Chapter 14. Analysis of a Solution of Two Acids
Objectives

- Use titration techniques to titrate a solution containing two acids with a standardized base.
- Using mole ratios of acid to base and the molarity of the standardized base, determine the concentrations of two acids, present in the same solution.
- Understand the relationship between the Ka, Kb, and Kw for weak acids and bases.
Introduction
Acid–base chemistry is fundamental to all life processes and we began our foundation of acids and bases early in the first chemistry course. We continue our discussion now by introducing a scale, pH, to quantify the strength of acids and bases. In lecture we are talking in depth about weak acids and weak bases and their relationship to water ionization. We have been familiarizing ourselves with new equilibrium constants Ka, Kb, and Kw.
Titration is a practical laboratory skill used to determine the concentration of ions in solution. We have performed an acid–base titration in the previous lab course. We will continue to perfect our titration techniques in this lab by titrating a solution containing two weak acids.
Discussion
20.1 Connection to Lecture
The pH of a solution at the equivalence point in an acid–base titration generally depends on the species present and on their concentrations. Consider a solution of nitrous acid.
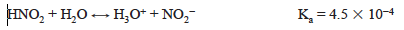
When nitrous acid is titrated with strong base, the titration reaction is
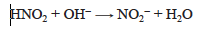
After a stoichiometric amount of standard base has been added to the nitrous acid solution, the resulting solution contains primarily nitrite ion, NO2−. The pH of the solution depends on the hydrolysis reaction or base ionization of the nitrite ion.
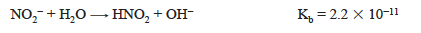
The value of Kb will be needed to calculate the pH and is obtained from Kw and Ka for nitrous acid.
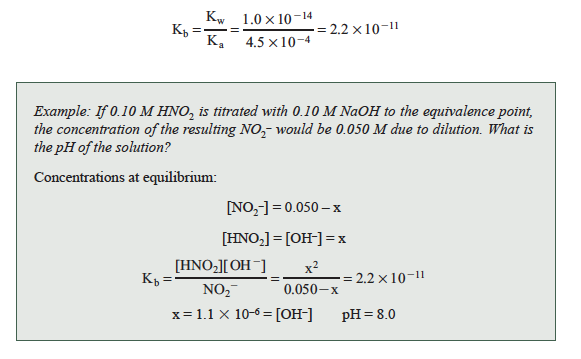
Titration of a solution of nitrous acid, using an indicator to signal the end point, requires an indicator that changes within one pH unit of the equivalence point. The indicator, cresol red, changes from yellow to red in the pH range of 7.0–8.8.
Question 20.1: Draw the titration curve in the graph below for the titration of HNO2 with NaOH indicating the equivalence point. Use arbitrary volumes of NaOH. Hint: What is the pH of the solution at the equivalence point?
If a solution contains two acids, the concentration of each can be determined in the presence of the other if the equivalence points have sufficiently different pH values, that is, if Ka values are significantly different for the two acids. Two different indicators are chosen, one for each end point. The solution you will be titrating contains two acids, H3PO4 and H2PO4−.
20.2 Titration of a Solution with Two Acids
The First Equivalence Point
Consider first a solution containing only H3PO4. Phosphoric acid is a triprotic acid that ionizes in a stepwise fashion.
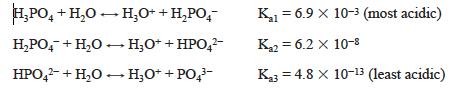
As NaOH is added to a solution of H3PO4, one proton is removed.
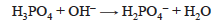
When an equivalent amount of OH– has been added, the solution contains primarily H2PO4−. The H2PO4– ion is a weak acid, so the weak acid ionization reaction is occurring.
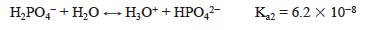
The dihydrogen phosphate ion is also the conjugate base of a weak acid, so the hydrolysis reaction is occurring.
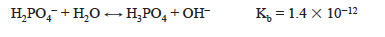
The value of Kb is calculated from Kw/Ka1. Since Ka2 > Kb, the solution is acidic. Specifically, the pH at the first equivalence point, when the solution contains primarily H2PO4−, can be approximated from the equation
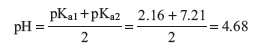
Bromocresol green changes from yellow to blue in the pH range 3.8–5.4 and is therefore a suitable indicator for the first end point. Through most of this range, the indicator will appear green. If a solution contains both H3PO4 and NaH2PO4, titration to the first end point, indicated by bromocresol green, will result in removal of one proton from H3PO4.
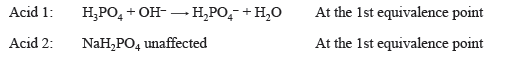
The NaH2PO4 originally present in the solution is unaffected, so the concentration of H3PO4 can be determined by this titration.
Data Analysis 1
From the equation for Acid 1 above, 1 mole of H3PO4 is reacted with 1 mole of NaOH, at the first equivalence point in this acid–base titration.

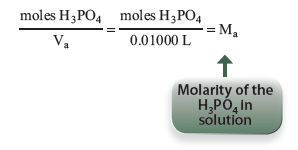
The moles of base can be calculated from the volume and molarity, and this will be the moles of H3PO4:
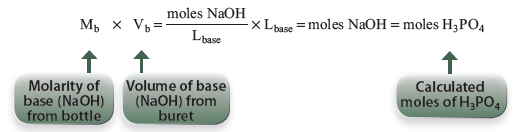
At the first end point in this experiment, H3PO4 is the only acid that has been titrated. The molarity of H3PO4 can therefore be determined by titrating to the first end point. The molarity of the acid, Ma, may then be calculated by dividing the moles of acid by the volume of acid, Va, you pipetted into the titration flask.
The Second Equivalence Point
If addition of NaOH is continued, the second proton is removed, quantitatively producing HPO42− at the second equivalence point.
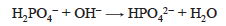
The HPO42− ion is again both a weak acid and the conjugate base of a weak acid.
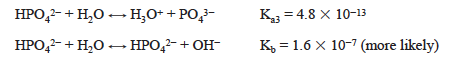
where Kb = Kw/Ka2. In this case, Kb > Ka3 so the solution is basic. The pH is approximated as follows:
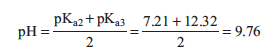
Either thymol blue, which changes from yellow to blue (8.0–9.6), or phenolphthalein, which changes from colorless to red (8.2–10.0), can be used for the second end point, though neither is ideal.
Question 20.2: Draw the titration curve for H3PO4 in the graph below showing the two equivalence points. Use arbitrary volumes of NaOH. Hint: What are the pHs of the solution at the equivalence points?
Titration to the second end point converts both of the initial acids, H3PO4 and H2PO4−, to HPO42−. Starting with a new sample, two protons are removed from the H3PO4 and one from the H2PO4−.
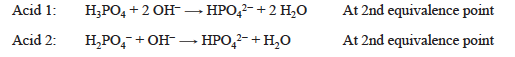
This titration allows calculation of the concentration of the H2PO4−, after adjusting for the amount of OH− consumed by the known amount of H3PO4 present.
Data Analysis 2
At the second end point, both of the acids in the solution have been converted to HPO42−.
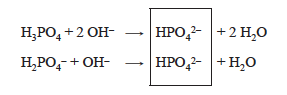
In the first acid, two protons have been removed for each mole of H3PO4 originally present.
For the salt, NaH2PO4, one proton has been removed.
Remember that the acid and salt are in the same solution so Va is equal to Vs. Total moles of acid (protons) in the solution equals moles of acid, a, plus moles of acid salt, s.
And moles of acid = moles of base, so:
The only unknown is Ms which can be calculated using the average molarity of the acid, obtained from the titration to the first end point and the volume of base used in titrating to the second end point.
Materials Required
Equipment
- two 125-mL Erlenmeyer flasks
- 25-mL buret
- 10-mL pipet
- 5-mL pipet
- pipet bulb
- 10-mL graduated cylinder
- buret clamp, ring stand
Chemicals
- Standardized 0.05xxx M sodium hydroxide, NaOH
- 0.1 M sodium dihydrogen phosphate, NaH2PO4
- 0.1 M disodium hydrogen phosphate, Na2HPO4
- bromocresol green solution
- thymol blue solution
- phenolphthalein solution
- unknown acid/salt solution
Common Equipment
- pH indicator color chart
Cautions
Goggles must be worn at all times.
Procedure
All buret readings should be recorded to the nearest 0.01 mL. Answer questions in your lab notebook as you go along. Discussions with your peers and TA are encouraged.
- Clean a buret with soap and water. Rinse well with distilled water. Drain out the water and rinse the buret with 5 mL of the standardized NaOH solution. Fill the buret with the NaOH solution. Record the molarity of the NaOH written on the label (4 significant figures).
- Prepare a reference solution by adding 5 drops of bromocresol green solution and 30 mL of 0.1 M NaH2PO4 solution to a 125-mL Erlenmeyer flask.
Question 20.3: What is the pH of this solution? What color should it be?
- Pipet 10.00 mL of your unknown sample into a 125-mL Erlenmeyer flask. Add 10 mL of distilled water and 5 drops of bromocresol green solution to the flask.
- Record the initial buret reading in your notebook. Titrate with the standardized 0.05xxx M NaOH solution until the shade of the color of the unknown solution matches the reference solution. Use a white background for all titrations. Record the final buret reading.
- Repeat steps 3 and 4 until two titrations agree within 0.1 mL.
- Prepare a reference solution by adding 5 drops of your second indicator solution and 25 mL of 0.1 M Na2HPO4 solution to a 125-mL Erlenmeyer flask.
Question 20.4: What is the pH of this solution? What color should it be?
- Pipet 5.00 mL of your unknown sample into a 125-mL Erlenmeyer flask. Add 10 mL of distilled water and 5 drops of your second indicator solution to the flask.
- Record the initial buret reading in your notebook. Titrate with the standardized NaOH solution until the shade of the color of the unknown solution matches the reference solution. Record the final buret reading.
- Repeat steps 7 and 8 until two titrations agree within 0.1 mL.
Waste Disposal
All solutions may be rinsed down the drain.
Points to Consider
In this lab you used titration techniques to determine the molarity of two acids in one solution.
- Turn in the report sheet with the lab report along with the sample calculations.
- Report the average molarities for each acid in solution and discuss any discrepancies between individual trials.
- Mention the appearance of each indicator at their respective end points. Why were two different indicators used and what criteria was used to determine this?
- Were there any errors associated with this lab and what impact did they have on your results?