Chapter 14. Voltaic and Electrolytic Cells
Objectives

- Learn about voltaic cells and electrolytic cells and describe the similarities and differences between them.
- Describe the key components and flow of electrons in electrochemical reactions.
- Understand what the Ecell of a reaction indicates and how this relates to the equilibrium expression and thermodynamics.
Introduction
Electrochemistry is the study of the relationships between chemical reactions and electricity. Your laptop, iPad, and cell phone use electrochemical reactions to function; without electrochemistry none of these devices would have the portability that we take for granted. Engineers, chemists, and materials scientists diligently search for ways to improve technology to make these devices lighter, faster, and more compact. They also look to improve their environmental impact and lessen the cost.
Building on thermodynamic concepts, we may determine the spontaneity of electrochemical reactions and learn how to manipulate these reactions to make them work. Using electricity as a driving force we can also make electrochemical reactions run uphill (in the nonspontaneous direction). This process, which is called electrolysis, has a variety of practical applications. One of the most important is electroplating, where electrolysis is used to deposit a thin layer of metal on a surface. Chrome plating is commonly used in the automobile industry to increase surface hardness, provide corrosion resistance, and deliver spectacular finishes. This lab has two parts—in the first part you will construct voltaic cells and measure the direction and flow of electrons; in the second part you will construct an electrolytic cell and explore the relationship between current, time, and the amount of metal produced.
Discussion
23.1 Connection to Lecture
In lecture we are learning about chemical reactions that involve oxidation and reduction. We have developed rules to help us properly balance electrochemical equations and keep track of our electrons. It is important that we fully understand these fundamental concepts so we may apply them in the laboratory.
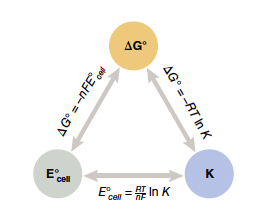
In the last lab we learned about Gibbs free energy and used this term to indicate if a reaction would occur spontaneously or nonspontaneously. In electrochemistry we again come back to the idea of spontaneity. If we place metals in solutions containing ions, and connect the metals with an external circuit, spontaneous redox reactions will occur at each piece of metal electrode and electrons will flow from one electrode to the other. Using concepts learned in lecture we may predict the direction the electrons will flow and the electric potential that develops, Ecell. Not surprisingly, Ecell, also called the cell potential, tells us about the spontaneity of a reaction. Experimentally we may find the Ecell by measuring the voltage of a cell using a multimeter. You will do this in the first portion of the lab and you will verify that the cell potential of a spontaneous reaction is a positive value. This E°cell is related to Gibbs free energy (ΔG°):
In this equation n is the number of moles of electrons and F is Faraday’s constant, 1 F = 96,485 C/mol = 96,485 J/V×mol. The ° indicates reactants and products are in their standard states (gases at a pressure of 1 bar ≈ 1 atm, dissolved substances present at concentrations of 1 M). Since a positive E°cell value corresponds to a spontaneous reaction, we see that a positive E°cell will produce a negative ΔG°, as expected for a spontaneous reaction.
Recall that in the last chapter we related Gibbs free energy to the equilibrium constant, K:
Then by substitution
With temperature measured in Kelvin, the (RT/nF) term is positive, so the sign of E°cell depends upon ln K. Remember that a large K corresponds to a reaction favoring the products, or a spontaneous reaction. The ln K of a large K value is a positive term. The opposite case, a reaction favoring the reactants, will have a small K, less than 1, a negative E°cell value, and a positive ΔG°, indicating a nonspontaneous reaction. A summary of the relationship between E°cell, ΔG°, and K is shown in Figure 23.1.
23.2 Measuring the Voltage of a Voltaic Cell
A voltaic cell, also called a Galvanic cell, is a device in which the spontaneous transfer of electrons occurs through an external path. The oxidation and reduction parts of the reaction are physically separated into two compartments, the anode and the cathode. We can write the equations of these two half-reactions; the oxidation half-reaction occurs at the anode and the reduction half-reaction occurs at the cathode. The reaction that happens in a voltaic cell is a spontaneous reaction so ΔG° is negative and our E°cell will be positive. If we know something about how easily metals may be reduced we can predict the reactions that will occur at each electrode of a voltaic cell. This is done using reduction potentials, some listed in Table 23.1 below.
Table 23.1 Reduction potentials for some metals. The metals at the top have higher reduction potentials and are more easily reduced than those with lower or negative reduction potentials.
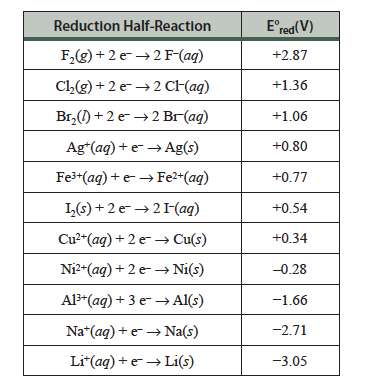
If we are given two half-reactions, we can determine which will be at the anode and which will be at the cathode by using the reduction potentials. For example, consider the two following reactions:
First of all it is important to remember that oxidation and reduction reactions must occur together. In this case that means that either:
The reduction half-reaction with the higher (more positive) E°red will preferentially be reduced. We see that both reduction potentials are negative, but the reduction potential for Ni2+(aq) is larger,1 so Ni2+(aq) ions will be reduced at the cathode. Thus Ca(s) will be oxidized at the anode. Using this knowledge we can write the half-reactions that occur at each electrode:
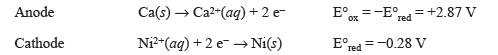
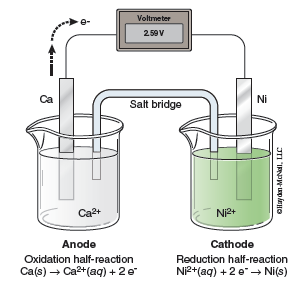
The E°cell of this reaction can be calculated with the following formula: E°cell = E°red(cathode) + E°ox(anode) = −0.28 V + 2.87 V = 2.59 V.2 A schematic representation of this voltaic cell is shown in Figure 23.2.
Question 23.1: Consider a voltaic cell constructed with a silver electrode placed in a 1.0 M AgNO3 solution and an aluminum electrode placed in a 1.0 M Al(NO3)3 solution. Write out the half-reactions that occur at the anode and the cathode. Determine the cell potential and the direction of electron flow.
In this lab you will construct a voltaic cell. Your experimental setup will look slightly different than Figure 23.2, as the anode and cathode compartments will be separated using a porous cup. The porous cup will allow the solutions to maintain neutrality, similar to the salt bridge seen in your notes and text. In order to measure the voltage of your cell you will need to obtain a multimeter from the stockroom. You will also need to clean your electrodes prior to using them. Once you obtain the cell potentials, E°cell, you will use the E°red potential for copper from the table to calculate your experimental E°red for each electrode. These will be added to the list of reduction potentials in Table 23.1.
23.3 Constructing an Electrolytic Cell
In the second part of the lab you will study an electrolytic cell. An electrolytic cell uses electrical energy to make the electrons run in the opposite direction than they do in a voltaic cell. We can think of the applied electrical voltage pushing the electrons uphill, much in the same way that a mechanical pump can push water uphill. Consider the example of an electrolytic cell involving Cu/Cu2+ in one compartment and Ag/Ag+ in the other compartment, as shown in Figure 23.3. From Table 23.1 we see that Ag+ ions have a larger reduction potential than Cu2+ ions, +0.80 V vs. +0.34 V, so in a voltaic cell we would expect the copper electrode to be oxidized and the electrons to flow through the external circuit to the silver electrode where Ag+ ions are reduced. In an electrolytic cell, electrical energy (a power supply) drives the electrons in the opposite direction, oxidizing silver and reducing Cu2+, as shown below.
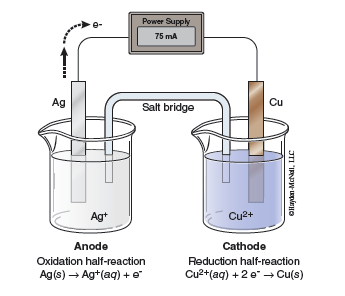
In this reaction electrons are transferred from copper to zinc ions. At the anode, silver metal loses electrons to form Ag+ ions. At the cathode, Cu2+ ions gain electrons to form copper metal.
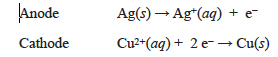
An external power supply pumps electrons out of the anode. As electrons are removed from silver atoms, Ag+ ions are formed. These electrons are pumped into the cathode and reduce the Cu2+ ions to form copper metal at the cathode.
Question 23.2: What would you expect to happen to the mass of the anode in an electrolytic cell? What would you expect to happen to the mass of the cathode in an electrolytic cell?
There is a quantitative relationship between moles of metal dissolved at the anode and moles of metal formed at the cathode. This is directly related to the moles of electrons transferred and we can say that:
Electrons carry an electric charge, which is measured in coulombs, C. The flow of charge is electric current and this is measured in amperes, amp. An ampere is a current of one coulomb per second, s.
An electron has a charge of 1.602 × 10−19 C; if we multiply by Avogadro’s number (6.022 × 1023 mol−1), we get the charge of 1 mole of electrons: 96,485 C/mol, a quantity called Faraday’s constant, F. In the electrolytic cell that you will construct in this lab, you will supply the cell with a specified current for a given amount of time. Knowing the current and time, and using Faraday’s constant, you can calculate the moles of electrons transferred in this reaction. For example, in the electrolytic cell shown in Figure 23.3, let’s say that we ran the power supply at 0.075 A for 6.00 minutes (360 seconds):
We can then go on and relate this quantity to the moles of each metal. In this example for every mole of electrons transferred we would dissolve (lose) 1 mole of silver from the anode and plate out (gain) 2 moles of copper at the cathode. Using atomic masses we can then convert from moles of each metal to the mass of each metal:
Mass Cu gained at cathode = 8.9 × 10−3 g Cu
Question 23.3 Metallic magnesium can be made in an electrolytic cell from an aqueous solution of magnesium chloride, MgCl2. In this process Mg(s) and Cl2(g) are produced. Which product is produced at the anode and which product at the cathode? How long would you have to pass a current of 2.5 A to produce 1.00 g of Mg?

Materials Required
Equipment
- Multimeter (BuckID required)
- two 100-mL beakers
- micropipet
- porous cup
- emery paper
- 100-mL and 10-mL graduated cylinders
- 75-mL and 25-mL test tubes
Chemicals
- 1 M copper(II) sulfate, CuSO4
- 1 M iron(II) sulfate, FeSO4
- 1 M lead(II) nitrate, Pb(NO3)2
- 1 M magnesium sulfate, MgSO4
- 1 M tin(II) chloride, SnCl2
- 1 M zinc sulfate, ZnSO4
- electrodes: Cu, Fe, Pb, Mg, Sn, Zn
- 3 M nitric acid, HNO3
- 3 M hydrochloric acid, HCl
- 6 M ammonia, NH3
- acetone, C3H6O
Common Equipment
- Kimwipes
Cautions
Lead, zinc, and salts of lead are toxic; wash your hands well after handling these chemicals. Goggles must be worn at all times.
Procedure
Answer questions in your lab notebook as you go along. Discussions with your peers and TAs are encouraged.
In this portion of the lab you will construct and measure the potential of voltaic cells. Work in teams of two. Each student will independently construct a total of three voltaic cells, each with copper as the cathode. Then share this data with your partner so you will have a total of six voltage readings for six voltaic cells.
- The multimeter is available for checkout at the stockroom window. A BuckID or driver’s license is required.
- Obtain a porous cup from your TA and place it in a clean 100-mL beaker. Fill the cup roughly 3/4 of the way with 3 M nitric acid. Then fill the beaker with distilled water. Allow the cup to soak while preparing the electrodes.
- Obtain a copper electrode and then three of the following electrodes: Cu, Fe, Pb, Zn, Mg, Sn. Have your partner obtain the copper electrode plus the other three electrodes so that between the two of you, you will construct these six cells: Cu/Cu, Cu/Fe, Cu/Pb, Cu/Zn, Cu/Mg, Cu/Sn.
- The surfaces of the electrodes are cleaned by washing in acid:For Cu, Sn, and Fe add approximately 20 mL of 3 M HCl to a 75-mL test tube. Drop the electrode into the test tube, and gently shake for 1 minute. Dispose of the acid in the drain and fill the tube with distilled water. Dispose of the water and dry the electrode with a Kimwipe.For the Pb electrode follow the same procedure, but use 3 M HNO3 and dispose of this in the inorganic waste beaker.For Mg and Zn add 1 mL of 3 M HNO3 to a 25-mL test tube and fill the test tube with distilled water. Immerse the electrode in the solution for 5 SECONDS, then rinse with water and blot dry. Dispose of the solution down the drain.
- Dump the contents of the porous cup and beaker down the drain, rinse the porous cup with water and shake to remove as much water as possible.
- Set up your voltaic cell by placing the porous cup in the 100-mL beaker. Fill the beaker with 1 M CuSO4 solution and place the copper electrode in the beaker.
- Place one of your electrodes in the porous cup. Fill the porous cup with the solution containing the same metal, as a cation, used for the metal electrode. (For example, if you are testing the Zn electrode you will use 1 M ZnSO4 solution.)
- To prepare the multimeter, use emery paper to sand the alligator clips. Plug the black wire into the common ground (COM) position on the front of the multimeter. Plug the red wire into the voltage (V) position on the front of the multimeter.
- Connect the black alligator clip to one electrode and connect the red alligator clip to the other electrode. Turn the knob on the multimeter to DC volts, which is indicated by a straight line above the dotted line on top of the letter V. After about 30 seconds record the voltage reading in your notebook. Switch the black and red alligator clips to the other electrode and record this reading.
Question 23.4: How do the two readings taken in step 9 compare to each other?
- Remove the porous cup, detach the alligator clip, wipe the electrode, and discard the solution down the drain. (Lead solutions should be disposed of in the inorganic waste beaker and not be dumped down the drain.)
- Rinse the porous cup with distilled water and then refill with a new electrode and corresponding sulfate solution. Attach the alligator clips so that the voltage reading collected is positive, and then record the reading. (Note: If the reading is negative, reverse the alligator connections.)
- Complete this again for your third and final electrode. Discuss with your partner your positive voltages and record each other’s readings.
- From Table 23.1 we know that the E°red of copper is 0.34 V. Using this value for your E°red(cathode), calculate the E°red(anode) for each of the other electrodes. Construct a table of reduction potentials like the one in Table 23.1 with these six metals.
E°cell = E°red(cathode) − E°red(anode)
- Clean the cup as in step 2 prior to starting Part B.
In this portion of the lab you will construct an electrolytic cell and measure the amount of a metal you plate onto an electrode. You will be able to compare this to the metal’s molecular weight. You must complete this section independently.
- Your TA will assign you a cathode and anode electrode (Cu/Cu, Pb/Pb, or Zn/Zn). Pick up the electrodes for your assigned cell. Properly prepare the electrodes, following step 4.
- Weigh each electrode to the nearest 0.0001 g and record their masses.
- Fill the 100-mL beaker with the cathode solution corresponding to your assigned cathode. Attach the alligator clip from your black wire to the cathode and place it in the beaker.
- Fill the porous cup with the solution corresponding to your anode, attach the red alligator clip to the anode, and place it in the porous cup. Place the porous cup into the beaker.
- Plug the power supply box into the multimeter by inserting the pins on the back of the power supply into the upper left/lower right holes on the multimeter.
- Adjust the knob on the multimeter to the mA position and turn the current switch to the ON position. Use the yellow button to select the DC setting and adjust the current knob to read as close to 75 mA as possible. It may take 30 seconds to stabilize the initial reading.
- In your notebook record the time when the current was turned on. While the current is passing through your cell, check the current every two minutes to be certain it is at 75 mA. Adjust as needed to keep the current as close to 75 mA as possible. If the current is deviating dramatically, record the value of the current every two minutes and then average this value for your current value.
- After 30 minutes stop the current. Record the time you stop the current. Disconnect the alligator clips.
- Carefully remove the anode, rinse it with distilled water and then acetone and allow to dry. While waiting for it to dry, prepare to weigh the cathode. If any small particles of metal are observed, add the particles to the appropriate electrode (more likely to occur for the cathode).
- Weigh a clean, dry watch glass and record its mass in your notebook. Carefully place the cathode on the watch glass and rinse it twice with water. After each rinse use a micropipet to remove as much water as possible, without removing any solid.
- Rinse the cathode twice with acetone. Again, remove as much acetone as possible with a micropipet without removing any solid. Set the watch glass containing the cathode aside to dry.
- While you are waiting for the electrodes to dry, soak the porous cup in 3 M nitric acid to clean, and then dispose of all solutions. Recall that any solutions containing lead must be collected in the inorganic waste beaker; all others may be rinsed down the drain.
- Once dry, weigh the electrodes and record the mass in your notebook. Return the electrodes to your TA.
- Calculate the atomic weight of each electrode.
- Neatly pack up the multimeter and return this to the stockroom to collect your ID.
Waste Disposal
All solutions containing lead must be collected and added to the inorganic waste beaker. All other solutions can be rinsed down the drain.
Points to Consider
- For this lab you should include the report sheet you generate containing: 1) a table of reduction potentials for the five electrodes you and your partner worked with, plus copper which is given, and 2) a table summarizing the electroplating data.
- Include the reduction potentials calculated for each of the electrodes in Part A. What do you notice about the Cu/Cu voltaic cell?
- Electroplating: Which electrode gained mass and which lost mass? What were the atomic weights calculated for each electrode? How do these compare to the one from the periodic table?