Chapter 14. Determination of the Molar Mass of an Unknown Acid
Objectives
- Prepare and standardize a base for use in titrations.
- Utilize acid–base titration concepts and laboratory techniques to determine the molar mass of an unknown acid sample based on whether it is monoprotic, diprotic, or triprotic.
Introduction
In this experiment you are asked to determine the possible molar mass of an unknown solid sample. The sample is an acid and soluble in water. How can you learn more about your sample? With a laboratory method called a titration. In this experiment you will perform several titrations and, along the way, become more skilled at calculations involving solution stoichiometry.
Titrations are an important and common laboratory method for quantitative chemical analysis. They allow a chemist to calculate the concentration of an unknown sample when it is reacted with a known concentration and amount of a standard solution. A standard solution has a precisely known concentration of an element or substance.
To be successful in this experiment you must work very carefully. Proper laboratory technique is especially important because your analysis depends on a series of steps, each performed correctly. This experiment requires an understanding of solution stoichiometry.
Discussion of Acids and Bases
An acid ionizes in aqueous solutions to form hydrogen ions, H+, also called protons. Since acids give away this proton they are also referred to as proton donors. Different acids can donate different numbers of protons. Hydrochloric acid, HCl, is a digestive fluid in your stomach. This compound has one hydrogen atom to donate:
Since it donates one proton per molecule of acid it is called monoprotic. An acid that can donate two protons is called diprotic. Sulfuric acid, H2SO4, is an example of a diprotic acid:
A base is a compound that will react with, or accept, a proton. When bases dissolve in water they produce hydroxide, OH− ions. The most common bases are soluble ionic comounds where the anion is a hydroxide group, like NaOH and KOH. When acids and bases are mixed together, the proton and hydroxide ion react with each other to form water in a neutralization reaction.
You can see from the molecular equation a salt, NaCl, is formed. In general a neutralization reaction between an acid and a base results in the formation of water and a salt. The net ionic equation for this reaction is simply:
In this example the hydrochloric acid and sodium hydroxide react in a 1:1 ratio.
Solution Concentration
When discussing solutions is it important to know how much solute is dissolved in the solvent, or its concentration. A common unit to describe solution concentration is molarity (symbol M), which is defined as the ratio of moles of solute per liter of solution:
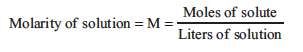
By manipulation of this simple relationship, one can also calculate the moles of substance in any volume of solution with a known concentration:
Question 5.1: How many moles of Ag are in 50 mL of a 0.436 M solution of Ag2SO4?
Titrations and Solution Stoichiometry
In a titration a substance in a flask reacts with a reagent added from a buret (Figure 5.1.) The substance in the flask is called the analyte (since it is being analyzed) and the solution in the buret is called the titrant. Often, the titrant is a standard solution that has a precisely known concentration.
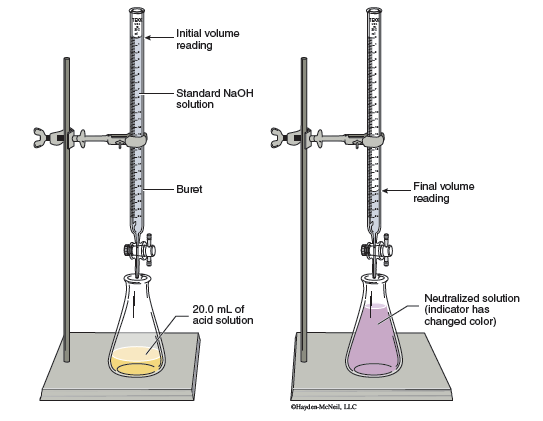
- There must be a well-defined and known reaction between the analyte and titrant.
- The reaction should be rapid and go to completion without interfering side reactions.
- There should be a marked change in some property of the solution when the reaction is complete.
- The point at which an equivalent or stoichiometric amount of titrant has been added is called the equivalence point. The point at which the reaction is observed to be complete is called the end point. The end point should closely coincide with the equivalence point.
- Finally, the reaction should be quantitative. If one knows the ratio for the moles of titrant reacting with moles of analyte, then either one of these (moles of titrant or analyte) can be calculated if the other is known.
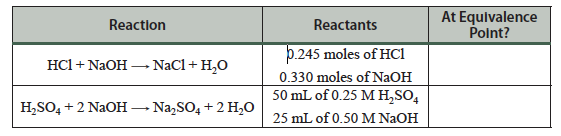
Question 5.2: Determine whether a given reaction is at the equivalence point:
The end point in an acid–base titration is usually identified by observing a color change due to the presence of an indicator, like phenolphthalein. As an example, consider the titration of HCl with the titrant NaOH. Prior to the titration the HCl is completely dissociated in solution and exists as H+(aq) and Cl–(aq) ions. When NaOH is added the acid and base react, producing Na+(aq) and Cl–(aq) ions; however, the solution is still acidic due to excess H+(aq). At the end point all of the HCl has been consumed and, due to the pH change, the phenolphthalein turns pink. With the continued addition of NaOH the pink color will deepen and the solution become basic with excess OH– ions.
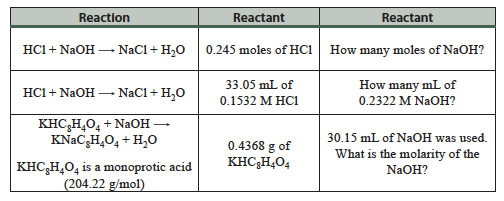
Question 5.3: Each of these reactions is at the equivalence point. How much of a particular reactant was used?
Finally, a titration provides a chemist with information regarding the equivalence point of a reaction. How is this information useful? Here are a few examples.
Example: A chemist does not know the concentration of a NaOH titrant solution, so they react it with a known amount of KHC8H4O4, a monoprotic acid. The exact mass of the acid is recorded (0.8985 g) and so the moles of acid are known. At the end point the volume of base is also known (which was 22.65 mL). The only remaining variable is the concentration of the base. How can the concentration be determined? Here are the experimental data and calculation:
Volume of NaOH to reach equivalence point = 22.65 mL = 0.02265 L
KHC8H4O4 + NaOH→ KNaC8H4O4 + H2O
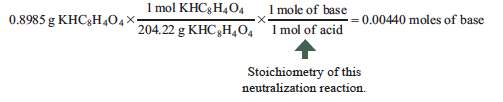
0.00440 moles of NaOH = molarity of NaOH × 0.02265 L
In this example, the concentration of NaOH is 0.1943 M.
Question 5.4: What is the concentration of a NaOH solution if it reacts with 0.7439 g of KHC8H4O4 and the equivalence point is reached after 19.23 mL of base has been added?
Example: A chemist has a NaOH standard solution with a precisely known concentration on 0.2349 M. This base is then reacted with 0.5543 g of a diprotic acid with an unknown molar mass and the equivalence point is reached after 29.92 mL. What is the molar mass? Here are the experimental data and calculation:
H2X + 2 NaOH → 2 H2O + 2 NaX
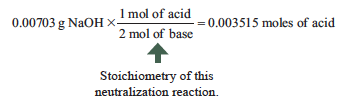
The molar mass is therefore 0.5543 g/0.003515 moles = 157 g/mol
Question 5.5: The equivalence point is reached when 0.7854 g of an unknown acid sample is reacted with 28.18 mL of a 0.2118 M NaOH solution. What is the molar mass of the acid, assuming it is monoprotic?
Overview of the Laboratory Procedure
In this experiment you will A) carefully determine the concentration of NaOH solution for use as standard solution, and B) use the standard solution in a titration to determine the molar mass of an unknown acid sample that may be monoprotic, diprotic, or triprotic.
As described above, this can be accomplished by reacting it stoichiometrically with a strong base, like NaOH. Although NaOH is good choice for a titrant, it is very difficult to prepare a sodium hydroxide solution with a precise concentration. Sodium hydroxide is extremely hygroscopic, meaning it readily absorbs moisture. This makes it virtually impossible to determine the initial mass of sodium hydroxide when the balance is open to the atmosphere. Fortunately, it is possible to precisely determine the concentration of a NaOH solution by titrating it versus a known mass the acid potassium hydrogen phthalate, KHC8H4O4, also known as KHP. KHP is a monoprotic acid that reacts with NaOH stoichiometrically. The end point in this reaction is evident based on a color change due to the indicator phenolphthalein. With this information the precise concentration of NaOH may be determined.
Precisely knowing the concentration of the NaOH is crucial for analyzing your unknown acid. You are asked to complete two trials; if they are not in close agreement you will need to complete additional trials. With the standard NaOH solution and your perfected laboratory skills the unknown acid will be analyzed. Once again, you should perform multiple trials and (of course) work carefully to minimize any errors.
Materials Required
Equipment
- 250-mL Erlenmeyer flask
- #6 rubber stopper
- 2–125-mL Erlenmeyer flasks
- 100-mL graduated cylinder
- 25-mL buret
Chemicals
- stock ~0.6 M sodium hydroxide, NaOH
- potassium hydrogen phthalate, KHC8H4O4 (KHP)
- phenolphthalein solution
- solid unknown acid sample
Common Equipment
- analytical balance
Cautions
Sodium hydroxide solutions are caustic. If your skin or clothing comes in contact with NaOH solutions, hold the affected area under running water.
Procedure
All buret readings should be recorded to the nearest 0.01 mL. Discussions with your peers and TAs are encouraged.
- Using a graduated cylinder, measure 200 mL of distilled water into a 250-mL Erlenmeyer flask. Add 40 mL of ~0.6 M NaOH stock solution to the distilled water. Stopper the flask well, and shake the flask to mix the solution. Keep the flask tightly stoppered while not in use.
- Clean a buret with soap and water. Rinse it well with distilled water and then with 5 mL of the dilute NaOH solution prepared in step 1. Fill the buret with the NaOH solution, and check for bubbles in the tip. Use a white background under the Erlenmeyer flask for all titrations..
- Tare a weighing dish and weigh about 0.35 g of KHP. Tare a clean 125-mL Erlenmeyer flask. Transfer the KHP into the flask and reweigh. Record the sample mass within 0.0001 g. Add about 30 mL of distilled water to the flask to dissolve the KHP. Add 3 drops of phenolphthalein solution.
- Record the initial buret reading in your notebook (remember a buret is read to the 0.01 mL). Titrate the KHP solution in the flask with the dilute NaOH solution until a faint pink color persists for 30 seconds. Make sure that all of the KHP has dissolved. Record the final buret reading.
- Calculate the molarity of the dilute NaOH solution.
- Repeat steps 3 through 5 until two values for the molarity agree within one percent. If the same mass of KHP is used, the volumes should agree within 0.1 mL.
- Tare a weighing dish and weigh between 0.10–0.12 g of your unknown acid sample. (If directed by your TA, use a higher mass.) Tare a clean 125-mL Erlenmeyer flask. Transfer the sample into the flask and reweigh. Record the sample mass to within 0.0001 g. Add 30 mL of distilled water to the flask—all of the sample may not dissolve. Add 3 drops of phenolphthalein.
- Refill the buret with the dilute NaOH solution that you standardized in part A. Record the initial buret reading in your notebook. Titrate with the standardized NaOH solution until a faint pink color persists for 30 seconds. Make sure that all of solid acid has dissolved—the salt formed in the titration is very soluble. Record the final buret reading.
- Calculate the molar mass of the acid assuming it is monoprotic.
- Repeat steps 7 through 9 until two values for the molar mass agree within one percent.
- Finally, calculate the molar mass of the unknown acid assuming it is diprotic, and once again assuming it is triprotic. These calculations should be included on your report form.
Waste Disposal
Vials containing excess KHP should be returned to your lab instructor. Excess unknown is collected in a weighing dish in the hood. Sign the waste disposal sheet. All solutions should be rinsed down the drain.