Chapter 14. Oxidation–Reduction Reactions of the Halogens
Purpose

To observe oxidation–reduction reactions of the halogens and to use these observations to identify a halogen-containing anion.
Discussion
Oxidation–reduction reactions involve electron transfer. As one substance loses electrons, another will gain those electrons. The loss of electrons is oxidation and the gain of electrons is reduction. The term redox is an abbreviated form of oxidation–reduction and implies the close connection between the two processes.
In considering oxidation–reduction reactions of the halogens, it is useful to recall the concept of electronegativity. Fluorine in compounds has the greatest ability to attract electrons to itself and is therefore the most electronegative element. The trend of decreasing electronegativity down a group is evident for the halogens. Electronegativities are
The relatively high electronegativities of the halogens compared to other elements (electronegativities increase from left to right across the periodic table) is the reason many reactions involving halogen-containing compounds result in formation of the element, X2, or a halide ion, X–.
One method of keeping track of electrons during oxidation–reduction reactions is to assign oxidation numbers to the elements. An oxidation number is the charge an element would have if both electrons in a bond are assigned to the more electronegative element. A set of rules for assigning oxidation numbers follows.
- The oxidation number of an element in its elemental (uncombined) form is zero. Elemental
forms of sodium, iron, oxygen, phosphorus, sulfur, and chlorine areNa Fe O2 P4 S8 Cl2
In each of these, the oxidation number of the element is zero.
- The oxidation number of a monatomic ion is the charge. Examples areNa+, +1 Fe3+, +3 O2–, –2 P3–, –3 S2–, –2 Cl–, –1
Notice that an oxidation number always has a sign that precedes the number. Since the Group IA and IIA metals in compounds are almost always present as the monatomic ions, the oxidation numbers for these elements are +1 and +2, respectively.
- Oxidation numbers of atoms in compounds add to zero. If the compound is ionic, and
you know the charge and therefore the oxidation number of one ion, the oxidation
number can be determined for the other. Chromium(III) chloride, CrCl3, contains chloride
ions with an oxidation number of –1. Oxidation numbers are on a per atom basis,
so the oxidation number of chromium must be +3. Using #Cr to stand for “oxidation
number of chromium,” an equation can be set up.#Cr + 3(–1) = 0#Cr = +3
Knowing the oxidation number of magnesium ion (+2), we can calculate the oxidation number of nitrogen in Mg3N2.3(+2) + 2(#N) = 02(#N) = – 6#N = –3
For binary compounds that are not ionic, the more electronegative element is assigned the oxidation number it would have in an ionic compound. Examples areCCl4 PF5 NO2 SO3
The oxidation number of the halogens in CCl4 and PF5 is –1. The oxidation number of C is therefore +4 and the oxidation number of P is +5. The oxidation number of oxygen in NO2 and SO3 is –2. The oxidation number of N is therefore +4 and of S is +6.
- The sum of oxidation numbers of atoms in polyatomic ions is the charge. An example
is the peroxide ion, O22–. The sum of oxidation numbers for the two oxygen atoms
is –2.2(#O) = –2#O = –1
The oxidation number of oxygen in peroxide is –1.
- The oxidation number of hydrogen is usually +1, of oxygen is usually –2, and of fluorine is always –1 in compounds. The exception for hydrogen occurs in combination with a metal in which hydrogen is the more electronegative element. Examples areNaH CaH2 ZnH2
Since metals in compounds always have positive oxidation numbers, hydrogen must be negative, –1. For oxygen, the exceptions occur in peroxides and superoxides. Examples of peroxides areH2O2 Na2O2 BaO2
Hydrogen peroxide is a molecular compound containing hydrogen in the +1 oxidation state and oxygen in the –1 oxidation state. Both Na2O2 and BaO2 are ionic peroxides containing the O22– ion as described on the previous page. Examples of superoxides are KO2 and CsO2. These are ionic compounds containing the O2– ion. The oxidation state of oxygen here is
During a chemical reaction, an increase in oxidation number for an element indicates that the element has lost electrons—oxidation. A decrease in oxidation number indicates reduction. Consider the following examples.
The 2+ charge on zinc in the products indicates that 2 electrons have been lost from the neutral atom in the reactants. The oxidation number has increased from 0 to +2; zinc has been oxidized. The Cu2+ ion gains two electrons to become Cu. The oxidation number of copper has changed from +2 to 0; copper ion has been reduced. Electrons have been transferred from zinc to copper ion.
The equation below represents the reaction between copper and nitric acid. Oxidation numbers are written above copper and nitrogen.

From the change in oxidation numbers, we see that copper has been oxidized and nitrogen has been reduced. Electrons have been transferred from copper to nitrogen.
The halogens are often found as the halide ion, X–, or in the –1 oxidation state. This is the most stable oxidation state. However, in combination with oxygen, the halogens are also found in positive oxidation states of +1, +3, +5, and +7. The elemental form is a diatomic molecule with an oxidation state of zero. With the large number of possible oxidation states, it is not surprising that oxidation–reduction reactions are important in the chemistry of the halogens.
We will limit our discussion and observations to the chemistry of chlorine, bromine and iodine.
The procedure has six parts:
- Displacement reactions of the halogens.
- Oxidation of halide ions by manganese(IV) oxide.
- Reactions of halide ions with sulfuric acid.
- Disproportionation reactions.
- Distinguishing between bromate and iodate ions.
- Identification of a halogen-containing anion.
A. Displacement reactions of the halogens.
The stability of the –1 oxidation state for the halogens indicates that the elements should readily accept electrons and be reduced. This tendency decreases down the group with decreasing electronegativity. Therefore, chlorine will take electrons from any halide ion below it in the periodic table.
If these reactions are performed in the presence of 1,2-dichloroethane, a nonpolar organic solvent, the product Br2 or I2 will dissolve more readily in the organic layer than in water. The identity of the halogen product can be determined by the color of the organic layer.
Bromine has a greater attraction for electrons than iodine.
These displacement reactions demonstrate the relative ability of the halogens to attract electrons.
B. Oxidation of halide ions by manganese(IV) oxide.
Several chemical oxidizing agents are capable of oxidizing chloride, bromide, or iodide ions to the elements. Reaction of manganese(IV) oxide with a halide salt is a laboratory method of preparation of the halogen. The reduction product of MnO2 in an acidic solution is Mn2+, an essentially colorless ion. The balanced equation for the reaction with chloride ion is
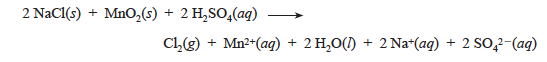
C. Reactions of halide ions with sulfuric acid.
The relative ease of oxidation of the halide ions depends on which ion is being oxidized: iodide is more easily oxidized (attracts electrons less strongly) than bromide which is more easily oxidized than chloride ion. Thus, a given reagent may oxidize iodide ion only, or iodide and bromide but not chloride. This is the case with sulfuric acid. Hot concentrated sulfuric acid can oxidize iodide and bromide ions but not chloride ion. The reaction of sulfuric acid with a metal chloride is a laboratory method of preparation of hydrogen chloride.
Analogous reactions of Br– and I2 with sulfuric acid cannot be used to produce HBr and HI because the halide ion is oxidized to the element.
An element that loses electrons readily is often capable of causing a greater degree of reduction than a more electronegative element. Thus, iodide ion causes greater reduction than bromide ion. When hot concentrated sulfuric acid reacts with halide ions, there are three possible reduction products under the conditions of this experiment. The sulfur, which is in the +6 oxidation state in H2SO4, can be reduced to SO2 (+4), S8 (0), or H2S (–2). Writing equations for these reactions, using X– and X2 to represent the halide ions and elemental halogen, results in the following.
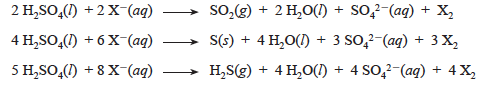
The sulfur-containing product which is actually formed can be determined by observation of properties. Both SO2 and H2S are gases which have very distinctive odors. Sulfur dioxide has a sharp, choking odor whereas hydrogen sulfide smells like rotten eggs. You should also observe that SO2 forms wispy white fumes in moist air. The observation of a light yellow solid indicates formation of sulfur as the reduction product. Note that you should observe a more reduced sulfur product as you go from bromide to iodide.
D. Disproportionation reactions.
A characteristic reaction of the halogens in intermediate oxidation states is disproportionation. This is a reaction in which the same element in a given substance is both oxidized and reduced. Bromine disproportionates in basic solution
Bromine has been oxidized from the zero to the +5 oxidation state and reduced from the zero to the –1 oxidation state.
The reaction is reversed (conproportionation) in acidic solution:
Analogous reactions occur with iodine.
E. Distinguishing between bromate and iodate ions.
Bromate ion and iodate ion react differently in acidic solution. In the presence of acid, bromate ion can oxidize water. The analogous reaction does not occur as readily with iodate ion. This difference in reactivity can be used to distinguish between bromate and iodate ions.
F. Identification of a halogen-containing anion.
Sodium or potassium salts of Cl–, Br–, I–, BrO3–, and IO3– are all white solids. The characteristic reactions described above can be used to identify these salts.
Prelaboratory Questions
Answer the assigned questions on a separate piece of paper.
- Assign oxidation numbers to the halogen in each of the following compounds or ions.
a. Br2O
b. HClO3
c. BrO2
d. Cl2
e. HClO2
f. KI
g. H5IO6
h. ClO– - Identify the element oxidized and the element reduced in each of the following
reactions.
a. Cl2(aq) + 2 I–(aq) → 2 Cl–(aq) + I2(aq)
b. 2 Br–(aq) + MnO2(s) + 4 H+(aq) → Br2(aq) + Mn2+(aq) + 2 H2O(l) c. 3 I2(aq) + 6 OH–(aq) → 5 I–(aq) + IO3–(aq) + 3 H2O(l)
d. 5 Br–(aq) + BrO3–(aq) + 6 H+(aq) → 3 Br2(aq) + 3 H2O(l)
- a. What would happen if chlorine water was added to an aqueous solution of sodium chloride?b. What would happen if chlorine water was added to an aqueous solution of sodium bromide?
- a. What would happen if MnO2 was mixed with KI and 9 M sulfuric acid?b. What would happen if MnO2 was mixed with KBrO3 and 9 M sulfuric acid?
Materials Required
Equipment
- five 4-mL test tubes
- microspatula
- medicine dropper
- 10-mL graduated cylinder
Chemicals
- solid sodium chloride, NaCl
- solid sodium bromide, NaBr
- solid potassium iodide, KI
- solid sodium bromate, NaBrO3
- solid potassium iodate, KIO3
- unknown sample
- chlorine water, Cl2(aq)
- bromine water, Br2(aq)
- 1,2-dichloroethane, C2H4Cl2
- solid manganese(IV) oxide, MnO2
- 9 M sulfuric acid, H2SO4
- concentrated (18 M) sulfuric acid, H2SO4
- 6 M sodium hydroxide, NaOH
Common Equipment
- none
Cautions
Both 9 M and concentrated sulfuric acid are corrosive. Chlorine, bromine, and iodine are toxic. 1,2-dichloroethane is a suspect carcinogen and may be irritating to the respiratory tract. Gaseous SO2 and H2S are possible reduction products of reaction of H2SO4 in part D. Sulfur dioxide is highly irritating to the respiratory tract and H2S is poisonous. Fume hoods must be on.
Procedure
- If available, use the plastic pipet in the cap of the chlorine bottle to add chlorine water directly in the following steps. Otherwise, half fill a 4-mL test tube with chlorine water and stopper it tightly for future use.
- Put a quantity of solid sodium chloride, about the size of a half-grain of rice, in a 4-mL test tube. Put similar quantities of solid sodium bromide in a second test tube, and solid potassium iodide in a third test tube.
- Add 1 mL of distilled water to each tube. Now add 10 drops of chlorine water and 1 mL of 1,2-dichloroethane to each tube. Stopper and shake the tubes. Record the color of both layers in each tube. Throughout the experiment 1,2-dichloroethane, which is used to dissolve the elemental halogens, Br2 and I2, will be referred to simply as dichloroethane. Dichloroethane is more dense than water, but can be less dense than salt solutions. The dichloroethane layer will be the more highly colored layer.
- Put a quantity of solid potassium iodide, about the size of a half-grain of rice, in a 4-mL test tube. Add 1 mL of distilled water, 10 drops of bromine water, and 1 mL of dichloroethane to the tube. Stopper and shake the tube. Record the color of both layers in the tube.
- Put a quantity of solid sodium chloride, about the size of a half-grain of rice, in a 4-mL test tube. Put similar quantities of solid sodium bromide in a second test tube, and solid potassium iodide in a third test tube.
- Put a similar quantity of manganese(IV) oxide in each test tube and mix the solids with a microspatula. Do not transfer solids between the tubes.
- Add 4 to 5 drops of 9 M sulfuric acid and 1 mL of dichloroethane to each of the three tubes. Record the color of both layers in the tube.
- Put a quantity of solid sodium chloride, about the size of a half-grain of rice, in a 4-mL test tube. Put similar quantities of solid sodium bromide in a second test tube, and solid potassium iodide in a third test tube.
- Add 4 to 5 drops of concentrated sulfuric acid to each of the test tubes. Note any color changes and signs of gas evolution. Gently blow across the top of each test tube and record your observations. If no fumes are observed, cautiously check the odor of any gas produced by fanning the fumes from the tube toward you.
- Mark three clean 4-mL test tubes BB, BI and II. Put a quantity of solid sodium bromide, about the size of a quarter-grain of rice, in the test tubes marked BB and BI. Put a similar quantity of solid potassium iodide in the test tube marked II. Add 1 mL of water and 2 to 3 drops of 9 M sulfuric acid to each test tube. Stopper and shake the test tubes to dissolve the solids.
- Add 2 mL of dichloroethane to each test tube and record any changes.
- Add a quantity of solid sodium bromate, about the size of a quarter-grain of rice, to the test tube marked BB. Add a similar quantity of solid potassium iodate to the test tubes marked BI and II. Stopper and shake the test tubes. Record any changes, noting the color of both layers. It may be helpful to centrifuge the test tubes to separate the layers.
- Remove and discard all of the less colored aqueous layer from each test tube using a medicine dropper. Add 5 to 6 drops of 6 M sodium hydroxide to each test tube, then stopper and shake the test tubes. Note any changes.
- Put a quantity of solid sodium bromate, about the size of a half-grain of rice, in a clean, dry 4-mL test tube. Put a similar quantity of solid potassium iodate in a second test tube.
- Add 4 to 5 drops of concentrated sulfuric acid to each of the test tubes. Note any color changes and signs of gas evolution.
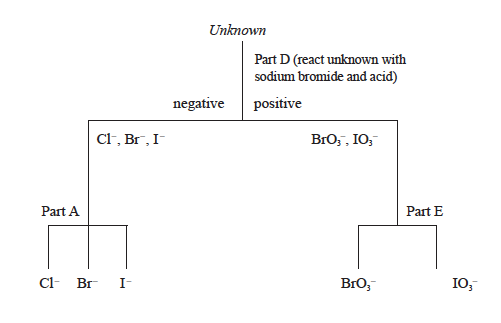
- Sodium or potassium salts of Cl–, Br–, I–, BrO3–, and IO3– are all white solids. Your lab instructor will give you an unknown sample. The characteristic reactions described above in parts A through E can be used to identify these salts, by substituting the unknown anion for the known anion in the appropriate tests. Parts A, B and C test for halides while D and E test for BrO3– or IO3–. The flowchart in Figure 8.1 is one possible sequence that could be used to identify the anion in your unknown salt. For example, adding the unknown to a test tube containing sodium bromide and sulfuric acid and dichloroethane (Part D) should give a color change only if the unknown is BrO3– or IO3–.
Waste Disposal
All solutions containing dichloroethane are disposed in the beaker for organic waste and neutralized with sodium bicarbonate. The waste disposal sheet should be properly filled in. Your lab instructor will dispose of the total volume in the appropriate container. Unused unknown may be rinsed down the drain.
Data Analysis
Explain how the results of your chosen tests support your conclusion about the identity of your unknown.