Energy Interaction Model
Energy Interaction Model
Chapter 2.
Energy Interaction Model
Energy Interaction Model
false
false

Algebraic Representations
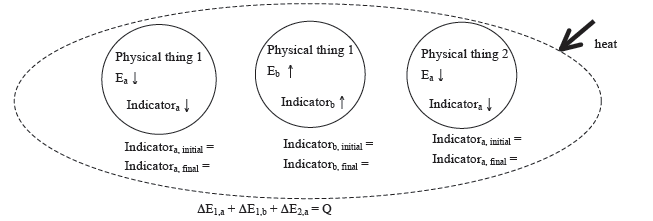
Closed system: ΔEtotal = ΣΔEi = ΔE1 + ΔE2 + ΔE3 + … = 0
Open system: ΔEtotal = ΣΔEi = ΔE1 + ΔE2 + ΔE3 + … = Q + W
Diagrammatic Representation
Generic Example involving two physical systems, three energy systems, and with Heat Input
Identification of beginning and end of interval:
beginning
end
Physical system:
Physical thing 1
Physical thing 2
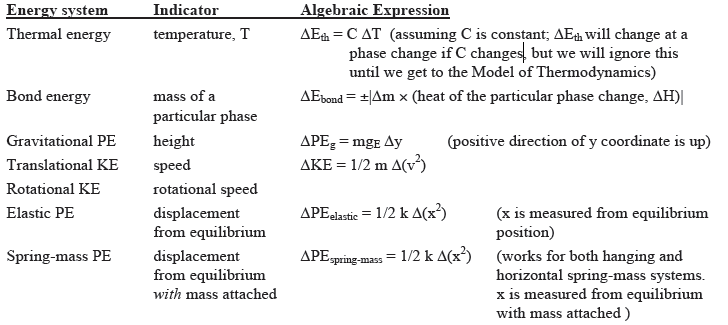
Commonly Used Energy Systems in Part 1
Useful Groupings of Energy Systems
Mechanical Energy
Internal Energy, U
Sum of kinetic and potential energies associated with the physical “objects.”
Internal Energy, U
Sum of kinetic and potential energies associated with the individual molecules/atoms
comprising a substance, as well as the energies associated with their atomic and
nuclear energies. We will mostly deal only with changes in the energies associated
with thermal and bond energies (chemical energies).