Chapter 2.
Energy-Interaction Model

Representations
Algebraic Representations
Diagrammatic Representation
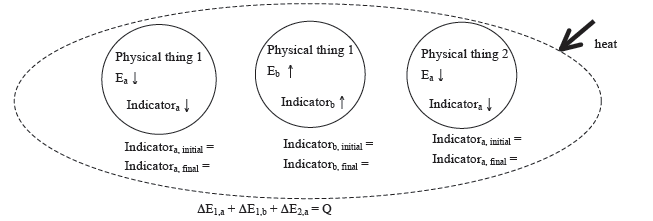
Generic Example involving two physical systems, three energy systems, and with Heat Input
Physical system:
Identification of beginning and end of interval:

Definitions of the Constructs of this Model
Energy
The scientific meaning of energy is rather tricky to convey in a sentence or two. There is a good reason for this: energy is an abstract concept that took scientists a long time to figure out and make sense of. Although the concept of energy is truly universal in the sense that energy changes are associated with nearly all interactions and processes, energy is not related to a single property of matter. For example, we all have an intuitive sense of “hotness” and we associate the concept of temperature with this property of matter. We associate the concept of force with the intuitive notion of push and pull. Energy, on the other hand, is associated with many properties or conditions of matter including temperature, force, motion, atomic level, mass, charge, and on and on. It is the fact that energy is so universal that makes it so difficult to define it precisely.
Another reason energy is difficult to pin down, is that the value of energy itself is seldom of importance; rather, it is the changes in the values of energy that seem to matter. In fact, we will see that change in energy is directly related to “how much” interaction occurred.
The following are useful statements about energy. Taken together they constitute a working definition of the construct (or concept of) energy. This concept will be developed further throughout the course.
Statements that help define energy
Energy is an abstract concept that characterizes the interactions of matter.
The change in energy of a physical system is a quantifiable measure of the degree of its interactions with other physical systems.
The importance of always being conscious of the word “change” as in the phrase “change in energy” and of the way this idea is represented mathematically cannot be overstressed. We signify “change in energy” mathematically by writing “∆E.” The uppercase Greek symbol “∆” often is used in science to indicate a change in some quantity. Does “∆E” look like “E” to you? No, of course not. Yet thousands of students have seriously messed up on exam questions because they don’t seem to “see” the difference between the following two expressions:
WRONG
Only one of the above statements makes any sense—the first one! But if we wrote the second one as:
RIGHT
WRONG
would make absolutely no sense. The point is, whether we are talking about a change in energy or the value of the energy matters a lot. You must be always conscious of which you are thinking about and why it is the one you want to think about!
Physical Systems
The word system is used in multiple ways in both scientific and non-scientific contexts. For example, we will talk about physical systems, particle systems, as well as energy systems, and doubtless a few other kinds of systems, and will, like other authors, not always include the modifier, but simply refer to “the system.” Make sure you understand how the word “system” is being used in particular discussions. In general, when we say physical system it means exactly what you would think it would mean: some stuff (objects, substances, apparatus, or just a beaker of water) that we wish to focus on. That is, we decide precisely specify what we have decided to include in the particular physical system we are talking about.
Energy Systems
Historically, many “forms” or “kinds” of energy have been identified. Sometimes, the form of energy labels the interaction that resulted in a change of energy, e.g., chemical, mechanical, or nuclear. It is often misleading to think of there being different forms, kinds, or types of energy, even though we have these “names of energy” in our vocabulary. Energy is energy, regardless of how it manifests itself. Thus, instead of speaking of forms of energy or kinds of energy, we speak of energy-systems. There is usually a one-to-one correspondence between an historically identified type or form of energy and what we will identify as an energy-system.
Our language encourages us to call the “thing” we refer to as an “energy system” more than just “an energy.” But it can be misleading to call it by any of the historical names that arose when scientists did not know that they were talking about the same thing. So in this course,we will usually refer to the total energy of a particular physical system as being the sum of the energies in the various energy systems belonging to that physical system. Likewise the change in energy of a particular physical system is the sum of the separate changes that occur in the various energy systems.
Energy is not a real thing that resides in physical systems, even though we often talk about it as if it were a “real thing.” Be careful here. Remember there is nothing at all physical about this “thing” we call energy, even when our language sometimes suggests that there is.
Interactions are modeled by treating energy as something that resides in (can be identified with) particular energy-systems. When interactions occur, energy-systems change. Frequently, it is useful to envision energy as being transferred into and out of particular energy-systems and from one energy-system to another as interactions occur. However, it is the changes in energy-systems (and not the particular transfers among energy-systems) that are the basis of the model.
When an interaction occurs, there is a change in one or more energy systems. For each energy system that changes, there is an observable (in the sense of being detectable) and quantifiable change in some part of the physical system. The magnitude of the change of each energy system is characterized by the change in its associated observable parameter. We will refer to this observable parameter as the indicator associated with a particular energy system. The questions of how to “divide up” the energy of some physical system and which energy-systems to include in the model get to the heart of the modeling process. The particular phenomenon under consideration will exhibit parameters that change as a result of the interaction or process. These parameters frequently turn out to be the indicators of the energy systems that need to be included in the particular model.
The word “energy” is sometimes used in everyday or non-scientific speech to mean something other than what we talking about here. The ideas mentioned in the previous paragraph can help us distinguish between the concept of “scientific” energy that is used in biology, chemistry and physics, from other uses of this same word. Specifically, if we can’t identify a quantifiable and consistent way to measure changes in what we are calling energy system when a process or interaction occurs, then the “thing” or concept that the word energy is applied to is not energy in the scientific sense.
Closed and open physical systems
There are two useful ways to express the principle of conservation of energy, one corresponding to closed physical systems and the other to open physical systems.
Closed physical system
In a closed physical system, there is no transfer of energy into (or out of) that physical system from some other physical system. (This condition would generally preclude mass transfers as well, since mass transfers would also result in energy transfers.) Another way to state the condition of being closed is that all interactions occur within the identified physical system. There are no interactions with other physical systems.
Open physical system
In an open physical system, there can be a net transfer of energy into (or out of) that physical system from some other physical system. Another way to state the condition of being open is that interactions can occur between matter in the identified physical system and matter in other physical systems (which might simply be the environment.
Energy transfers: Heat, Q & Work, W
Even though energy is not a physical thing that can be transferred, it is conventional and sometimes useful to model the process as if transfers of energy actually take place among various physical systems. When energy is transferred into a physical system from another physical system, it is customary to name the energy transferred as either heat, or work. The name heat, Q, is given to energy transfers that occur as result of a difference in temperatures. Energy “flows” from the physical system at the higher temperature to the physical system at the lower temperature. We will use the word “heat” only as a label for this kind of energy transfer . Note, however, that historically, the word “heat” was also used to mean what is now commonly called thermal energy. The notation ∆Q will never be used, since this implies a change in a state quantity, which Q is not. However, the notation dQ will be used to mean an infinitesimally small amount of energy transferred as heat.
When you are reading science journal articles or other textbooks, especially older ones, be sure you understand how the authors are using the word “heat.” This is important, because the concept of energy transfer as heat is very different from the concept of thermal energy. We (and most modern authors) now restrict the use of the word “heat” to its meaning as a transfer of energy between physical systems as a result of temperature differences.
The term work, W, is used to describe the energy transferred between physical systems (objects), which exert forces on one another and move relative to one another. Specifically, work is a transfer of energy into or out of one physical system by a force exerted by another physical system. The change in energy results from an interaction in which an object moves through a distance parallel to the force exerted on it. The physical system containing the object doing the pushing loses energy if the motion is in the direction of the push, while the physical system containing the object that is pushed, increases in energy by the same amount. Like heat, work is a scalar quantity; i.e., it has no direction associated with it.
Where the subscript “parallel” means to multiply only the part of the force and ∆x that are parallel to each other. Heat and work, which are energy transfers, are measured in the same units as is energy. In the SI system of units, joule, J, is the unit of energy, which according to the above relation for W must be equivalent to the product of the SI unit of force and distance. That is, a joule is equivalent to the product of the SI unit of force, newton, N, and the unit of distance, meter, m. That is, a joule, J is equivalent to a newton meter, N m.
One of the great advantages of the energy interaction model is that we don’t have to be concerned with the details of how energy transfers occur. We don’t need to have a microscopic explanation, for example, of how friction causes increases in thermal energy-systems and decreases in other non-thermal energy-systems. On a microscopic scale, all kinds of energy transfers are taking place between individual atoms and molecules. The energy transfers we are talking about that occur between different physical systems are always the net transfers that occur as a result of an interaction between those physical systems.
Processes and Interactions
One of the frequent ways beginning students go astray is to not be perfectly clear in their own mind how the beginning and end of a process is defined or determined. Sometimes, we think of an interaction instead of a process. The same problem arises. What determines the beginning of the interaction and what determines the end of the interaction?
Often there is a point in time that can be identified with the beginning and the end of the interaction. This is not usually an actual clock time, such as 10 minutes after one, but rather, something happens at a particular time. The something that happens can be easily pictured in our mind and remembered. It is when the “ball was let go” or “just before the ball hit the ground.” Or perhaps it was when the coffee pot was turned on and when the coffee pot was turned off. Something physically happens that we make a conscious decision to identify with the beginning of the time interval and something else happens that we use to identify with the end of the time interval that corresponds to the interaction or process we are interested in. It is crucial to always clearly identify these starting and ending “events,” which precisely determine the interval over which the process or interaction occurs. We often use phrases like, “Determine the interval.” to indicate precisely defining the beginning and end of a process.
State of a Physical System
Related to the discussion regarding the beginning and ending of a process or interaction is the idea also mentioned previously that we are not concerned about the details of the interaction in an energy conservation approach. In fact, all we care about is how the state of the system changed from the beginning to the end of the process or interaction. By state of the physical system we mean the values of certain parameters that changed. For the Energy-Interaction Model we care only about the indicators that tell us how much the energy changed in each energy system. This notion of state of the physical system will become more obvious as we work through more and varied phenomena using the Energy Interaction Model.
Energy Systems Related to Thermal and Chemical Processes
When dealing with thermal and chemical processes from a macroscopic perspective, it is convenient and useful to define energy systems that correspond to the empirically determined heats, i.e., the change in enghalpies, ∆H’s, that correspond to the process or interaction. These include heats associated with physical phase changes and with the formation of various molecular species. The indicator for an energy system associated with these processes would be the amount of substance that changed phase or for a chemical reaction, the amount of substance that was formed or that “disappeared” during the interaction.
The general form of the expression for the amount of energy change in these processes will be
The indicator for the energy change is the amount of substance that changed, ∆m. If ∆H, which is typically given as an intensive quantity, has units of J/kg, then ∆m will have units of kg. If ∆H is given as a molar quantity, with units of J/mol, then ∆m will have units of moles.
When there are no phase changes or chemical reactions occurring and heat is added to a substance, its temperature changes with the amount of change dependent on its heat capacity. The energy change associated with this process is exactly equal to the heat, Q, added, since we have stipulated that there are no other energy systems that are changing. From our discussion of heat capacity in the Three-Phase Model of Matter we had
Equating ∆E with Q and rearranging, we have an expression for the change in the energy system associated with this process:
Temperature is the indicator for this energy system.
These two energy systems we have just defined are very useful for two reasons. (1) They correspond to changes in indicators that we can directly observe and (2) depend on parameters, (∆H and C) that are tabulated for most substances. Additionally, as we have defined them, during a physical or chemical reaction, only one of them will be changing at a given time.
Both phase changes and chemical reactions involve the making and breaking of atomic and/or molecular bonds. That is, they are closely related to “bond energy.” When heat is added to a substance and its temperature changes, its “thermal energy” changes. Consequently, these two macroscopic energy systems we have defined above are closely related to bond energy and thermal energy that we will develop from a particle model later. Rather than use a different name for the energy systems involving the empirically determined parameters, ∆H and C, and macroscopic indicators, ∆m and ∆T, we will simply refer to them as the “bond energy system” and “thermal energy system,” remembering that after particle models for the bond and thermal energies are developed, our understanding of these macroscopic energy systems will be deepened and we will see how they are specifically related to the microscopically defined bond and thermal energies using particle models.
So, to summarize, our understanding now of the constructs bond energy system and thermal energy system is:
At a physical phase change, only the bond-energy system changes and the change is given by
Between phase changes, only the thermal-energy system changes and the change is given by
In chemical reactions, there will typically be several bond energy changes, corresponding to each of the molecular species present in either the reactants or products. The energy change for each reactant or product is given by
The temperature is assumed to have the same value after the reaction as it did before the reaction.
These energy systems, defined above, are the appropriate energy systems to use in the Energy-Interaction Model whenever we are using empirically determined values for the various ∆H’s and C’s. The salient features of the phenomenon that we want to model are precisely the ∆H’s and C’s characterizing the particular substance(s) and the indicators ∆m and ∆T. These salient features appear in both the phenomenon and in the particular model we construct. It is this particular model that we then use to create explanations and to make numerical predictions.
The algebraic signs of thermal and bond energies
It is very important to make sense of the algebraic sign of the change in the energy system based on what physically is happening. This is actually very simple to do, once you get the hang of it. However, it is easy to make a simple algebraic slip-up when actually calculating numerical values. You should always check to see if the final algebraic sign makes sense.
Any thermal energy-system for which the temperature increases during the process will always have a positive change in energy. Likewise, any thermal energy system for which the temperature decreases will always have a negative change in energy. This is consistent with the simple notion that thermal energy increases with increases in temperature, because at higher temperatures there is “more vigorous” motion of the particles.
Any bond energy-system for which bonds are broken during the process or interaction will always have a positive change in energy. Likewise, any bond energy-system for which bonds are formed during the process or interaction will always have a negative change in energy. This is consistent with the common experience of having to add energy (and thus increase the bond energy) to vaporize liquid water that is at 100 °C. The bonds that had existed in the liquid phase disappear (are broken) in the process of the liquid changing to a vapor. You will probably need to struggle mentally with this last point: broken bonds have more energy than intact bonds. Work on this until it “seems obvious” to you.
Commonly Used Energy Systems
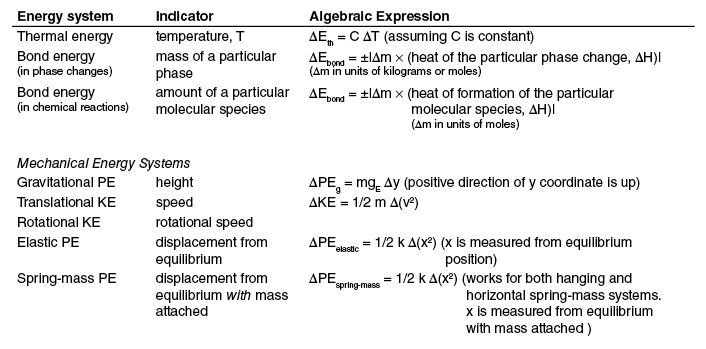
Useful Groupings of Energy Systems
Mechanical Energy
Sum of kinetic and potential energies associated with the physical “objects” as a whole, not with the internal energies of the objects.
Internal Energy, U
Sum of kinetic and potential energies associated with the individual molecules/atoms comprising a substance, as well as the energies associated with their atomic and nuclear energies.
Meaning of the Model Relationships
- The heart of the Energy-Interaction Model is energy conservation, one of a small number of powerful conservation principles used throughout science. One way of expressing a conservation principle is that for an isolated physical system there are certain physical properties that do not change during an interaction or process. A process or interaction is determined by explicitly indicating the start and end points of the process or interaction; i.e., identifying the beginning and end of the interval that defines the process or interaction. The power of a conservation principle is that values of state variables (variables like P, V, T, and n for a gas) need to be known at only these two particular times (the ends of the interval), and not at intermediate times during the interval during which the process or interaction takes place.
In particular, we need only know the values of the indicators of the various energy systems at the ends of the interval. It is these values that allow us to calculate actual energy changes. - The total energy of every physical system can be expressed as a sum of the energies of separately identifiable energy systems. This division of the energy into energy systems can be carried out in multiple ways. The energy associated with a particular energy system can be expressed in terms of an observable and measurable property of the physical system. We call these properties indicators. The change in energy of each energy system can be determined from the observed change in the indicator that occurs from the beginning to the end of the time interval during which the interaction takes place.
- Conservation of energy in a closed physical system (isolated with respect to energy transfers from other physical systems):
The total energy of that physical system must remain constant during the interaction or process. When internal interactions occur this conservation principle can be expressed in terms of changes of energy systems: the changes of the energies of all energy systems associated with that physical system must sum to zero.
The sum of the increases in energy of those energy-systems that experience an increase exactly equals the sum of decreases of the energy-systems that experience a decrease. When we express this mathematically using the “∆” symbol, a decrease in energy will result in a negative value for the change. So another way of stating conservation of energy is to say that the sum of all the energy changes is zero. - Conservation of energy in an open physical system:
During an interaction or process during which energy is added or removed from the physical system as heat or work, the changes in energy of all energy systems associated with that physical system must sum to the net energy added (or removed) as heat and/or work. Equivalently, the change in the total energy of that physical system must equal the net energy added (or removed)
Energy System Diagrams
Energy-system diagrams illuminate the types of energy transformations that occur when two or more physical systems interact, or when a constraint is removed and changes occur in two or more energy systems within a single physical system. The diagram helps make clear the physical systems involved, the particular energy-systems involved, and the changes in those energy-systems resulting from the interaction. The initial and final states of the systems should be clearly indicated on the diagram. These diagrams are “before-to-after” diagrams. That is, they indicate the state of the systems before the interaction occurs and the state of the systems after the interaction has occurred. (Note that there is one diagram that includes both the initial and final states. The focus is the change that occurs because of the interaction.) We use energy-system diagrams because they are useful. They help us to systematically apply the energy conservation principle to a particular physical situation using the Energy-Interaction Model.
Drawing Energy-System Diagrams When Modeling the Interaction as a CLOSED System
Listed here are the conventions we adopt in this course.
- The beginning and ending of the interaction is specified by explicitly writing down a condition of the physical system that corresponds to the beginning and end of the time interval over which the interaction occurs. These times are referred to as the initial and final times.
- The specific energy-systems that changed during the specified time interval are indicated by circles and labeled sufficiently to identify the energy-system.
- If transfers of energy to the environment are significant, due to friction, for example, include the thermal system of the environment on the energy-system diagram. That is, enlarge the boundary of the closed system to include the environment.
- The change in energy of each energy-system, whether an increase or decrease, is indicated, when known, with an “up” or “down” arrow.
- Changes in the observable parameter (indicator) associated with each energy-system that occur as a result of the interaction should be shown. If the quantitative change in the value is known, it should be given. If not, an “up” or “down” arrow can be used following the symbol of the indicator to indicate an expected increase or decrease.
Drawing Energy-System Diagrams When Modeling the Interaction as an OPEN System
Listed here are the conventions we adopt in this text.
The important difference between open and closed systems is that energy from outside the open physical system can be transferred into or out of the physical system as heat, Q, or work, W. (By definition, these transfers do not occur for a closed system.) The only difference, then, in the energy-system diagrams for the two types, is that the diagram for an open system needs to explicitly show the transfer of Q and/or W. This is done by drawing a dashed oval around all of the energy-systems (to indicate the open physical system boundary) and drawing arrows terminating on the boundary to show a Q or a W transfer.
Generic Example of an Energy System Diagram involving two physical systems, three energy systems, and with Heat Input
Physical system:
Identification of beginning and end of interval:


Comments:
- What is shown in the diagram above is the minimum that must always be written down. Most of the hard thinking will have been done to get to this point. Often, many explanations of physical phenomena can be constructed using this diagram without going further and substituting in explicit expressions for the individual change in energy terms and numerical values for various parameters. Even if you are required to continue the process through to a numerical result, you must do the mental work of constructing the energy-system diagram to this point prior to doing any numerical calculations.
- In an open-system diagram, the arrow showing energy transfer into the system is drawn in the direction of the actual energy flow and labeled with the words “heat” or “work,” and not “Q” or “W.” This is to preserve the standard convention of calling Q and W positive when energy is entering the physical system. Therefore, equations expressing conservation of energy are always written as shown in the diagram, with Q and W always written on the right side of the equation (without a negative sign, even if energy is leaving the physical system). When numerical values are substituted in for Q or W, those values will be written with a negative sign, if energy is leaving the physical system.
General Process of Constructing an Energy-System Diagram
Listed here are some general questions you need to ask yourself as you use the Energy-Interaction Model. The order suggested is logical, but it is often necessary to cycle back to previous questions. The energy-system diagram is a tool to help you use the Energy-Interaction Model. The energy-system diagram helps you keep track of the many important details you need as you construct the particular model corresponding to the particular physical situation you are interested in.
- What happened? State the essence of the physical phenomenon of interest in your own words. You don’t need to write this down, but you should have an “internal dialogue” with yourself.
- What is the boundary of the physical system you are modeling? Answer this question by listing the physical things you intend to include in the physical system. (Examples of “physical things” are: air, H2O, hand, heatpack, “all of the chemicals”.) Energy systems are always energy systems of particular physical things, so the energy systems you identify (in step (4)) will depend on the physical things you decide to include within the boundaries of your overall physical system. In some cases, it might be useful to identify two or more separate physical systems, each with its own energy-system diagram.
- What is the extent of the process or interaction? This means identifying the beginning and end of the time interval corresponding to the process/interaction that you defined and explicitly writing this on the diagram.
- What energy systems do you include in your diagram? Answer: Which indicators are changing? Each indicator that changes corresponds to an energy system that changes. Put these energy systems into your diagram as labeled circles (for example, “H2O Ethermal”). Include the indicator for each energy system inside the labeled circle with an up or down arrow showing whether the indicator increased or decreased during the process, if known. Show increases and decreases in each energy system as up or down arrows next to the abbreviation for the energy system, if known. What you are doing here is picking out salient features from the particular instance of a physical phenomenon that relate to energy to include in the particular model you are constructing in order to answer questions, develop explanations, make numerical predictions, etc. related to the particular phenomenon you are interested in.
- What are the values of the indicators at the times corresponding to the ends of the time interval you chose in step (3)? Record the initial and final values of the indicators next to their respective energy systems. Remember that all of the initial and final values of indicators should correspond to the same initial and final times. (Note: It is not always necessary to identify specific initial and final values for all indicators. Sometimes, depending on the question, you only care about the change in the indicator (e.g., ΔT). At other times, you may only know that the final value of the indicator is greater than (or less than, or equal to) the initial value. The point is not to memorize a series of steps, but to be as specific as possible about what you know. The diagram is not an end in itself, but a tool to lead you through your analysis. The diagram helps you connect the particular physical phenomenon to the particular model you are constructing.)
- Is the physical system in your particular model open or closed? If you are modeling the phenomenon as an open physical system, draw a dashed oval enclosing all of the energy systems, and use an arrow that stops or starts on the oval to show heat or work entering or leaving the physical system. You may find it necessary to go back to step (2) and modify the boundary of the physical system.
- Write an equation expressing energy conservation for your particular energy-system diagram, in terms of the ΔE’s. Each term in your conservation of energy equation must correspond to an energy system in your diagram.
Units for Energy
The historical development of the energy concept separately as heat and mechanical energy, as well as the widespread use of several different systems of units, has created a multitude of energy units. But, energy is energy and all forms, types, whatever, can and should be expressed in the same basic energy unit. Fortunately, this is now becoming common. The SI unit of energy is the joule, J, (rhymes with cool). All other energy units are related to the joule through an appropriate conversion factor.
A concept closely related to energy is power–the time rate of energy change or energy transfer. The SI unit of power is a joule per second and is given the name watt, W.
Fortunately, essentially everyone within the scientific and technical community has now embraced the International System (SI) system of units. We will generally use SI units in this course. However, in those instances where non-SI units are commonly used, we will use both, and expect you to be able to convert back and forth. An advantage of working exclusively in SI is that you don’t have to be concerned about unit conversions (and keeping track of them).
SI base units
The SI base units for mass, length, and time are kilogram (kg), meter (m), and second (s). Other SI units can be expressed in base units when desired. For example, a joule is a kg m2/s2.
SI unit of temperature
The kelvin (K), the SI unit for temperature, is another independent base unit. The zero of the kelvin scale is at thermodynamic zero, the so-called “absolute zero” of temperature. (Note that “kelvin” is used without the word “degree” attached.) Although the zero of the Celsius, or centigrade, scale is not at absolute zero, the kelvin is the same size as the Celsius degree. Thus, when dealing with temperature differences, it is sometimes convenient to use Celsius degrees for ∆T. For example, the SI unit for specific heat, J/kg K is equal to (and will sometimes be written as) J/kg C°.
SI Units related to energy:
Some common energy units and conversions to SI:
1 kWh = 3.6 MJ
1 erg = 10–7 J
1 cal = 4.184 J
1 food Calorie (big “C” calorie) = 1 kcal = 4.184 kJ
1 ft•lb = 1.36 J
1 eV = 1.602 x 10–19 J
1 BTU = 778 ft•lb = 252 cal = 1.054 kJ
Videos
A Look at Conservation of Energy from Hayden-McNeil on Vimeo.
Thermal Energy and Bond Energy from Hayden-McNeil on Vimeo.
Check Your Understanding of the Basics
Practice Question 1
Liquid water at 100°C boils to produce water vapor at 100°C. Choose the physical system to be all the water molecules.
Before doing an energy analysis, we need to choose a definite physical system whose energies we will follow. What should we choose as our physical system (and write at the top of our energy-interaction diagram)?
After choosing the physical system we need to define a beginning time and an ending time. What should we choose (and write on the timeline).
Practice Question 2
Liquid water at 100°C boils to produce water vapor at 100°C. Choose the physical system to be all the water molecules.
From the description of this interaction given above, decide whether bond energy is increasing, decreasing, or not changing. Show the direction of energy change by drawing the appropriate arrow where indicated on a separate sheet of paper. If there is no change then you can write Δ(Bond E) = 0. Click submit to see the Discussion.
Practice Question 3
Liquid water at 100°C boils to produce water vapor at 100°C. Choose the physical system to be all the water molecules.
From the description of this interaction given above, decide whether thermal energy is increasing, decreasing, or not changing. Show the direction of energy change by drawing the appropriate arrow where indicated on a separate sheet of paper. If there is no change then you can write Δ(Thermal E) = 0. Click submit to see the Discussion.
Practice Question 4
Liquid water at 100°C boils to produce water vapor at 100°C. Choose the physical system to be all the water molecules.
From the description of this interaction given above, decide whether energy was being added or removed from the system as heat. On a separate sheet of paper, show the direction of energy transfer with the appropriate direction of the arrow sketched above the word “Heat”. Click submit to see the Discussion.
Practice Question 5
Liquid water at 100°C boils to produce water vapor at 100°C. Choose the physical system to be all the water molecules.
From the description of this interaction given above, what can you say with absolute certainty about the relative magnitudes of the energy changes of any energy systems and the energy transfers and explain how this is shown on the energy-system diagram.
Practice Question 6
Photosynthesis in a plant happens according to the following:
sunlight + 6CO2(g) + 6H2O(l) = C6H12O6(aq) + 6O2(g).
Before doing an energy analysis, we need to choose a definite physical system whose energies we will follow. What should we choose as our physical system (and write at the top of our energy-interaction diagram)?
After choosing the physical system we need to define a beginning time and an ending time. What should we choose (and write on the timeline).
Practice Question 7
Photosynthesis in a plant happens according to the following:
sunlight + 6CO2(g) + 6H2O(l) = C6H12O6(aq) + 6O2(g).
Choose the physical system to be all the C, H, and O atoms (including the ones that are bound into molecules etc.). We are interested in which of the various energies changed and by how much.
Give a reason for the direction of the change, if any, of each of the two bond energy systems shown in the energy-system diagram. On a separate sheet of paper, show these arrows on the energy-system diagram. Click submit to see the Discussion.
Practice Question 8
Photosynthesis in a plant happens according to the following:
sunlight + 6CO2(g) + 6H2O(l) = C6H12O6(aq) + 6O2(g).
Choose the physical system to be all the C, H, and O atoms (including the ones that are bound into molecules etc.). We are interested in which of the various energies changed and by how much.
Give a reason for the direction of the change, if any, of the thermal energy system of all the atoms. On a separate sheet of paper, show the appropriate arrow on the energy-system diagram. Click submit to see the Discussion.
Practice Question 9
Photosynthesis in a plant happens according to the following:
sunlight + 6CO2(g) + 6H2O(l) = C6H12O6(aq) + 6O2(g).
Choose the physical system to be all the C, H, and O atoms (including the ones that are bound into molecules etc.). We are interested in which of the various energies changed and by how much.
Is energy entering or leaving the system? On a separate sheet of paper, show the appropriate arrow on the energy-system diagram. Click submit to see the Discussion.
Practice Question 10
Photosynthesis in a plant happens according to the following:
sunlight + 6CO2(g) + 6H2O(l) = C6H12O6(aq) + 6O2(g).
Choose the physical system to be all the C, H, and O atoms (including the ones that are bound into molecules etc.). We are interested in which of the various energies changed and by how much.
a) What can you say about the relative sizes of any energy changes based on the completed energy-system diagram you just made?
b) Which group of molecules, i.e., the reactants or the products, has the bigger change in bond energy?