Chapter 1. An Acid-Base Titration: Determining Molecular Weight
Discussion
Objectives
- Prepare and standardize a base for use in titration; understand the need for standardizing and the significant figures obtained using the buret and pipet.
- Utilize acid-base titration concepts and laboratory techniques to determine the molecular weight of an unknown acid by titration with a standardized base.
- Be able to describe where accurate solution measurements are needed and how these are used to calculate the molecular weight.
Connection to Lecture
In lecture you have been discussing acids and bases and the reactions between them. You have also been learning about chemical solutions and their concentrations. In this lab you will explore acid-base chemistry and perform a titration to determine the concentration of hydroxide ions in a solution and then use that solution to determine the molecular weight of an unknown acid.
An acid ionizes in aqueous solutions to form hydrogen ions, H+, or protons. Since acids give away protons they are also referred to as proton donors. Different acids can give away different numbers of protons. Hydrochloric acid, HCl, is the main fluid in your stomach, has one hydrogen atom and can donate it, shown by the equation below:
HCl(aq) → H+ (aq) + Cl−(aq)
Since it gives away just one proton per molecule of acid it is called monoprotic. An acid that can give away two protons is called diprotic, and one that gives away three or more is polyprotic.
A base is a compound that will react with, or accept a proton. When bases dissolve in water they produce hydroxide, OH−, ions. Many bases will have OH groups in their formula, though some do not.
Credit: PhET, Colorado University (www.phet.colorado.edu)
Titrations of acids and bases
A titration is an experimental method to determine the concentration of an ion in solution. This uses a reaction between the ion we are investigating with an ion of known concentration, or standard. Titrations are common for several reactions including precipitation reactions and acid-base reactions, the latter you will investigate in this lab.
In an acid-base titration, we utilize the neutralization reaction to determine our ion concentration. We know from lecture that NaOH and HCl will react to form water and NaCl(aq).
Molecular equation
HCl(aq) + NaOH(aq) → H2O(l) + NaCl(aq)
Net ionic equation
H+(aq) + OH−(aq) → H2O(l)
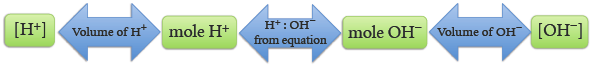
From the molecular equation we note that one mole of HCl requires one mole of NaOH to be neutralized. From the net ionic equation we see that one mole of H+ requires one mole of OH− to be neutralized, and vice versa. This mole ratio between H+ and OH− allows us to use a titration to determine the ion concentration. If we know the concentration of one ion and how much of it we need to neutralize the other ion, we can then simply calculate the concentration of the other ion.
1 mole H+ = 1 mole OH−
While doing the titration we have a flask of ions, OH− for this example, with an unknown concentration, Figure 5.1(a). H+ ions (the titrant) of a known concentration are added slowly from the buret (b). These H+ ions combine with the OH− ions in the flask to form H2O. Once all of the OH− ions are used up, we stop adding the H+ ions (c) and this is known as the equivalence point. We will add a color indicator, phenolphthalein, to help us see the end point; at the end point the solution will turn from clear to pink.

At the equivalence point a stoichiometric amount of the standard solution (H+ in our example) has been added. When using the buret for the titration it is important to titrate slowly once you get close to the endpoint. The end point is close when the solution starts to turn pink when titrant is added, but then turns clear again once the flask is swirled. Having a white paper underneath the flask will help you see the color more clearly. To titrate slowly, add the titrant one drop at a time and swirl the flask after until the faint pink color remains. Once the endpoint is reached the buret reading is taken and the volume of the titrant needed is known.
We use this volume to calculate the number of moles of H+ that were added. Using the stoichiometric relationship the number of moles of OH− in the sample may be calculated. Then, using the volume of the original NaOH sample, the [OH−] may be calculated, Figure 5.2.
Titration in this lab and data analysis
In order for the titration to be successful we must have a solution of known concentration and we must be able to easily determine the volume that we add. From lab 1 we know that we may use a buret to accurately determine the volume of titrant. The solution of known concentration is called the standard solution. The standard solution may be prepared in three ways:
- The solid solute may be weighed, dissolved in water and diluted to a known volume.
- A concentrated solution of known concentration can be diluted to a known volume.
- A solution of approximately the desired concentration can be prepared by either of the first two methods and then the concentration can be determined by reacting it with a known amount of primary standard.
The last process is the one we will do in Part A of this lab. The primary standard is a solid of high purity that can be dried to a constant weight. The primary standard you will use if potassium hydrogen phthalate, KHC8H4O4, abbreviated KHP. Potassium hydrogen phthalate is an acid salt with one ionizable proton. It reacts with a strong base as follows:
HC8H4O4(aq)+ OH−(aq) → HC8H4O42_ + H2O(l)
This reaction is performed as a titration with a carefully weighed sample of KHP in the titration flask and the hydroxide solution to be standardized in the buret. (Note that the standard and unknown have been switched here.) At the equivalence point
moles of ionizable H+ = moles of OH−
We will add the indicator, which will change from clear to pink once neutralization has been reached. Moles of ionizable H+ are known from the mass and molecular weight of the KHC8H4O4.
1.
What is the molecular weight of KHP?
| A. |
| B. |
| C. |
| D. |
The volume of the base has been measured so the molarity of the base, [OH−], may be calculated.
= mol/L of standard NaOH = [OH−]
We have now standardized our NaOH base and may use it in Part B to do the titration. The titration for this lab will include an unknown monoprotic acid of unknown concentration using the NaOH standardized solution.
2.
What is the ratio of moles of H+ to moles of OH− if we are using
NaOH and an unknown monoprotic acid?
| A. |
| B. |
| C. |
| D. |
The NaOH will be added slowly to a solution of the unknown acid until the indicator turns a faint pink color, Figure 5.3a (middle). The buret will be read at this point, the endpoint, giving the volume of OH−.


The moles of base can then be calculated using the molarity of the base determined in Part A and the volume of it we used for the titration in Part B.
Since we are told that the unknown acid is monoprotic we know that
moles of ionizable H+ = moles of OH−
The molecular weight of the acid is then calculated from grams of our unknown sample and moles of H+ of our unknown sample.
3.
How many significant figures should your reported NaOH molarity and molecular weight have? (Hint: how many significant figures do all of your recorded masses and volumes have?)