6.5 The Search for the Biological Basis of Memory
KEY THEME
Early researchers believed that memory was associated with physical changes in the brain, but these changes were discovered only in the past few decades.
KEY QUESTIONS
How are memories both localized and distributed in the brain?
How do neurons change when a memory is formed?
Does the name Ivan Pavlov ring a bell? We hope so. As you should recall from Chapter 5, Pavlov was the Russian physiologist who classically conditioned dogs to salivate to the sound of a bell and other neutral stimuli. Without question, learning and memory are intimately connected. Learning an adaptive response depends on our ability to form new memories in which we associate environmental stimuli, behaviors, and consequences.
Pavlov (1927) believed that the memory involved in learning a classically conditioned response would ultimately be explained as a matter of changes in the brain. However, Pavlov only speculated about the kinds of brain changes that would produce the memories needed for classical conditioning to occur. Other researchers would take up the search for the physical changes associated with learning and memory. In this section, we look at some of the key discoveries that have been made in trying to understand the biological basis of memory.
The Search for the Elusive Memory Trace

An American physiological psychologist named Karl Lashley set out to find evidence for Pavlov’s speculations. In the 1920s, Lashley began the search for the memory trace, or engram—the brain changes that were presumed to occur in forming a long-term memory (see photo caption). Guiding Lashley’s research was his belief that memory was localized, meaning that a particular memory was stored in a specific brain area.
memory trace or engram
The hypothetical brain changes associated with a particular stored memory.

Lashley searched for the specific location of the memory trace that a rat forms for running a maze. Lashley (1929) suspected that the specific memory was localized at a specific site in the cerebral cortex, the outermost covering of the brain that contains the most sophisticated brain areas. Once a rat had learned to run the maze, Lashley surgically removed tiny portions of the rat’s cortex. After the rat recovered, Lashley tested the rat in the maze again. Obviously, if the rat could still run the maze, then the portion of the brain removed did not contain the memory.
Over the course of 30 years, Lashley systematically removed different sections of the cortex in trained rats. The result of Lashley’s painstaking research? No matter which part of the cortex he removed, the rats were still able to run the maze (Lashley, 1929, 1950). At the end of his professional career, Karl Lashley concluded that memories are not localized in specific locations but instead are distributed, or stored, throughout the brain.
Lashley was wrong, but not completely wrong. Some memories do seem to be localized at specific spots in the brain. Some 20 years after Lashley’s death, psychologist Richard F. Thompson and his colleagues resumed the search for the location of the memory trace that would confirm Pavlov’s speculations.
Thompson classically conditioned rabbits to perform a very simple behavior—an eye blink. By repeatedly pairing a tone with a puff of air administered to the rabbit’s eye, he classically conditioned rabbits to blink reflexively in response to the tone alone (Thompson, 1994, 2005).
Thompson discovered that after a rabbit had learned this simple behavior, there was a change in the brain activity in a small area of the rabbit’s cerebellum, a lower brain structure involved in physical movements. When this tiny area of the cerebellum was removed, the rabbit’s memory of the learned response disappeared. It no longer blinked at the sound of the tone. However, the puff of air still caused the rabbit to blink reflexively, so the reflex itself had not been destroyed.
Thompson and his colleagues had confirmed Pavlov’s speculations. The long-term memory trace of the classically conditioned eye blink was formed and stored in a very localized region of the cerebellum.
So why had Karl Lashley failed? Unlike Thompson, Lashley was working with a relatively complex behavior. Running a maze involves the use of several senses, including vision, smell, and touch. In contrast, Thompson’s rabbits had learned a very simple reflexive behavior—a classically conditioned eye blink.
Thus, part of the reason Lashley failed to find a specific location for a rat’s memory of a maze was that the memory was not a single memory. Instead, the rat had developed a complex set of interrelated memories involving information from multiple senses. These interrelated memories were processed and stored in different brain areas. As a result, the rat’s memories were distributed and stored across multiple brain locations. Hence, no matter which small brain area Lashley removed, the rat could still run the maze. So Lashley was right in suggesting that some memories are distributed throughout the brain.
Combined, the findings of Lashley and Thompson indicate that memories have the potential to be both localized and distributed. Very simple memories may be localized in a specific area, whereas more complex memories are distributed throughout the brain. A complex memory involves clusters of information, and each part of the memory may be stored in the brain area that originally processed the information (Greenberg & Rubin, 2003).
Adding support to Lashley’s and Thompson’s findings, brain imaging technology has confirmed that many kinds of memories are distributed in the human brain. When we are performing a relatively complex memory task, multiple brain regions are activated—evidence of the distribution of memories involved in complex tasks (Frankland & Bontempi, 2005; Khan & Muly, 2011).
The Focus on Neuroscience below describes a clever study that looked at how memories involving different sensory experiences are assembled when they are retrieved.
The Role of Neurons in Long-Term Memory

MYTH  SCIENCE
SCIENCE
Is it true that all memories, even complex ones, are located in a single part of the brain?
What exactly is it that is localized or distributed? The notion of a memory trace suggests that some change must occur in the workings of the brain when a new long-term memory is stored. Logically, two possible changes could occur. First, the functioning of the brain’s neurons could change. Second, the structure of the neurons could change. Given those two possibilities, the challenge for memory researchers has been to identify the specific neurons involved in a given memory, a task that is virtually impossible with the human brain because of its enormous complexity. What this task required was a creature with a limited number of neurons that is also capable of learning new memories.
Enter Aplysia, a gentle, seaweed-munching sea snail that resides off the California coast. The study of Aplysia over the past 30 years has given memory researchers important insights into the brain changes involved in memory. Why Aplysia? Because Aplysia has only about 20,000 good-sized neurons. That was a key reason why memory researcher Eric Kandel (2006, 2009) chose this unassuming creature to study the neuronal changes that occur when a new memory is formed for a simple classically conditioned response.
FOCUS ON NEUROSCIENCE
Assembling Memories: Echoes and Reflections of Perception
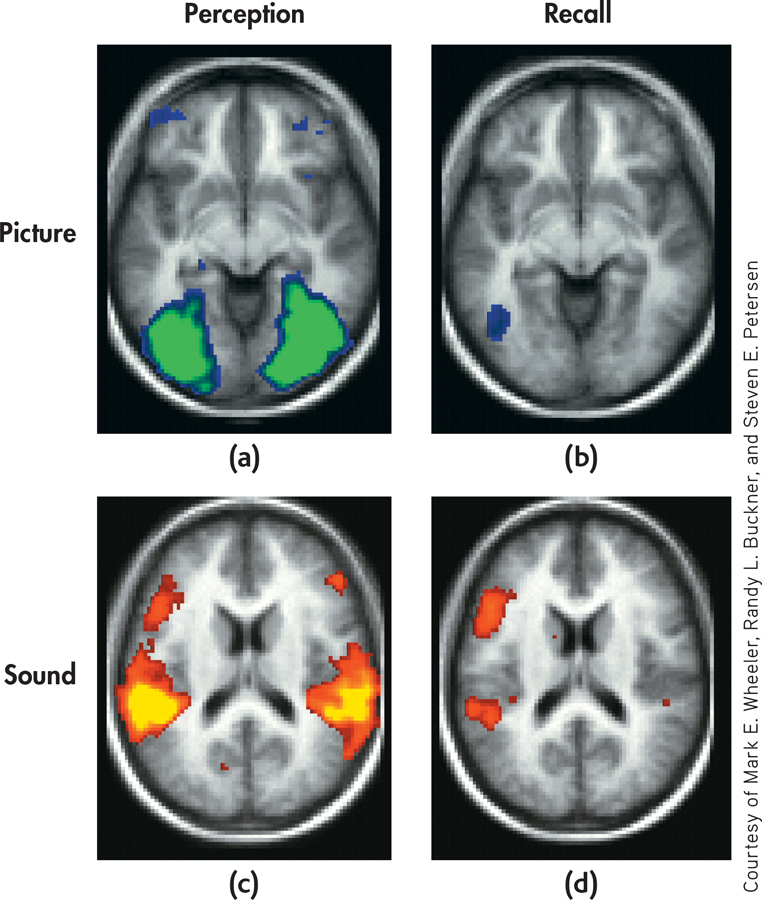
If we asked you to remember the theme from Sesame Street, you would “hear” the song in your head. Conjure up a memory of your high school cafeteria, and you “see” it in your mind. Memories can include a great deal of sensory information—sounds, sights, and even odors, textures, and tastes. How are such rich sensory aspects of an experience incorporated into a memory that is retrieved?
Researchers set out to investigate this question using a simple memory task and fMRI (Wheeler & others, 2000). Participants studied names for common objects that were paired with either a picture or a sound associated with the word. For example, the word “dog” was either paired with a picture of a dog or the sound of a dog barking. The researchers then used fMRI to measure brain activity when the volunteers were instructed to recall the words they’d memorized.
The results? Retrieving the memory activated a subset of the same brain areas that were involved in perceiving the sensory stimulus. Participants who had memorized the word dog with a picture of a dog showed a high level of activation in the visual cortex when they retrieved the memory. And participants who had memorized the word dog with the sound of a barking dog showed a high level of activation in the auditory cortex when they retrieved the memory.
Of course, many of our memories are highly complex, involving not just sensations but also thoughts and emotions. Neuroscientists assume that such complex memories involve traces that are widely distributed throughout the brain. However, they still don’t understand how all these neural records are bound together and interrelated to form a single, highly elaborate memory (Khan & Muly, 2011).
If you give Aplysia a gentle squirt with a WaterPik, followed by a mild electric shock to its tail, the snail reflexively withdraws its gill flap. When the process is repeated several times, Aplysia wises up and acquires a new memory of a classically conditioned response—it withdraws its gill when squirted with the WaterPik alone. This learned gill-withdrawal reflex seems to involve a circuit of just three neurons: one that detects the water squirt, one that detects the tail shock, and one that signals the gill-withdrawal reflex (see FIGURE 6.10).
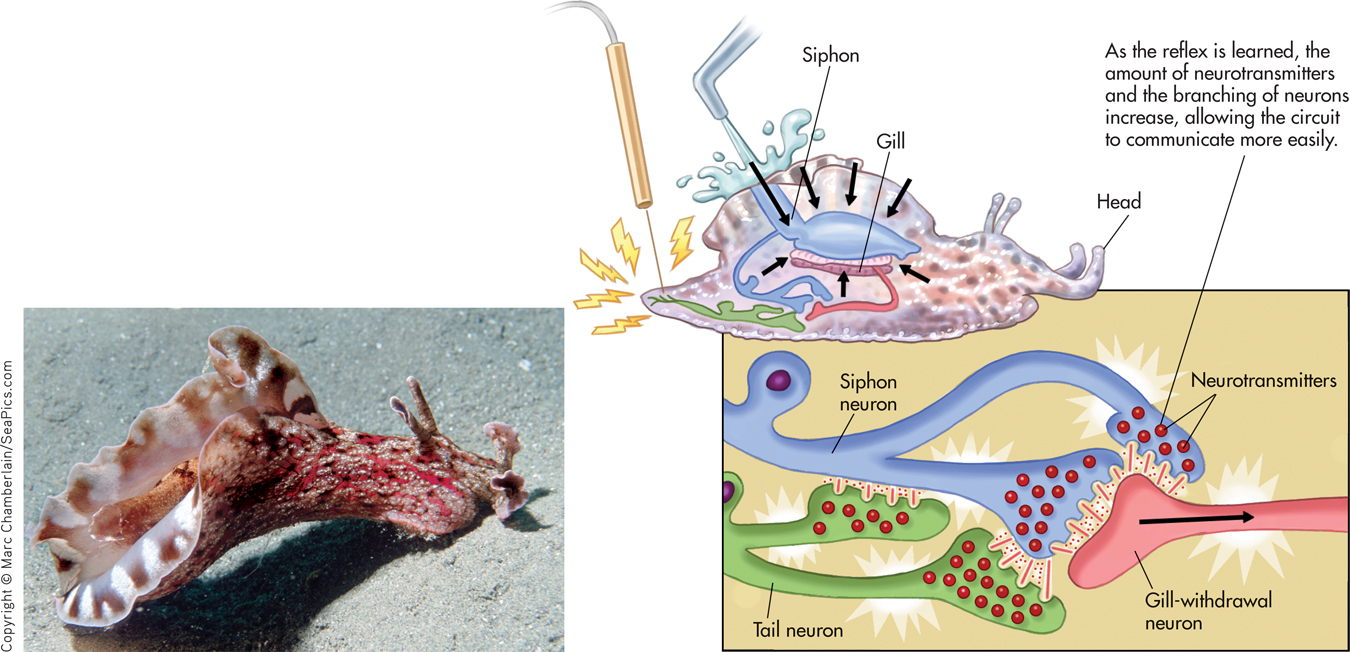
When Aplysia acquires this new memory through repeated training trials, significant changes occur in the three-neuron circuit (Kandel, 2001). First, the function of the neurons is altered: There is an increase in the amount of the neurotransmitters produced by the neurons. Second, the structure of the snail’s neurons changes: The number of interconnecting branches between the neurons increases, as does the number of synapses, or communication points, on each branch. These changes allow the neurons involved in the particular memory circuit to communicate more easily. Collectively, these changes are called long-term potentiation, which refers to a long-lasting increase in synaptic strength (Baudry & others, 2011; Lisman & others, 2012).
long-term potentiation
A long-lasting increase in synaptic strength between two neurons.
The same kinds of brain changes have been observed in more sophisticated mammals. Chicks, rats, and rabbits also show structural and functional neuron changes associated with new learning experiences and memories. And, as you may recall from Psych for Your Life in Chapter 2, there is ample evidence that the same kinds of changes occur in the human brain.
In terms of our understanding of the memory trace, what do these findings suggest? Although there are vast differences between the nervous system of a simple creature such as Aplysia and the enormously complex human brain, some tentative generalizations are possible. Forming a memory seems to produce distinct functional and structural changes in specific neurons. These changes create a memory circuit. Each time the memory is recalled, the neurons in this circuit are activated. As the structural and functional changes in the neurons strengthen the communication links in this circuit, the memory becomes established as a long-term memory (Kandel, 2006, 2009).
CONCEPT REVIEW 6.3
Remembering Famous Names
Match each of the following names with one of the descriptions below:
Question
George Sperling Elizabeth Loftus Hermann Ebbinghaus Karl Lashley Richard F. Thompson Eric Kandel | The psychologist who was one of the first people to scientifically study memory and forgetting The psychologist who succeeded in identifying the specific brain location of a simple classically conditioned response The neuroscientist who won a Nobel Prize for his success in determining the neural basis of memory in Aplysia One of the most widely recognized experts on memory distortions, eyewitness testimony, and false memories The psychologist who tried but failed to find the specific location of individual memories in the brain The psychologist who identified the duration of visual sensory memory |
Processing Memories in the Brain
CLUES FROM AMNESIA
KEY THEME
Important insights into the brain structures involved in normal memory have been provided by case studies of people with amnesia caused by damaged brain tissue.
KEY QUESTIONS
Who was H.M. and what did his case reveal about normal memory processes?
What brain structures are involved in normal memory?
What are dementia and Alzheimer’s disease?
Prior to the advent of today’s sophisticated brain imaging technology, researchers studied individuals who had sustained a brain injury or had part of their brain surgically removed for medical reasons. Often, such individuals experienced amnesia, or severe memory loss. By relating the type and extent of amnesia to the specific damaged brain areas, researchers uncovered clues as to how the human brain processes memories.
amnesia
(am-NEE-zha) Severe memory loss.
RETROGRADE AMNESIADISRUPTING MEMORY CONSOLIDATION
One type of amnesia is retrograde amnesia. Retrograde means “backward moving.” People who have retrograde amnesia are unable to remember some or all of their past, especially episodic memories for recent events. Retrograde amnesia often results from a blow to the head. Boxers sometimes suffer such memory losses after years of fighting. Head injuries from automobile and motorcycle accidents are another common cause of retrograde amnesia. Typically, memories of the events that immediately preceded the injury are completely lost, as in the case of accident victims who cannot remember details about what led up to the accident.
retrograde amnesia
Loss of memory, especially for episodic information; backward-acting amnesia.

Apparently, establishing a long-term memory is like creating a Jell-O mold—it needs time to “set” before it becomes solid. This process of “setting” a new memory permanently in the brain is called memory consolidation (Dudai & others, 2011). More specifically, memory consolidation is the gradual, physical process of converting new long-term memories to stable, enduring memory codes (Medina & others, 2008). If memory consolidation is disrupted before the process is complete, the vulnerable memory may be lost (Dudai, 2004).
memory consolidation
The gradual, physical process of converting new long-term memories to stable, enduring memory codes.
In humans, memory consolidation can be disrupted by brain trauma, such as a sudden blow, concussion, electric shock, or encephalitis (Riccio & others, 2003). Similarly, many drugs, such as alcohol and the benzodiazepines, interfere with memory consolidation. In contrast, stimulants and the stress hormones that are released during emotional arousal tend to enhance memory consolidation (Nielson & Lorber, 2009).
ANTEROGRADE AMNESIADISRUPTING THE FORMATION OF EXPLICIT MEMORIES
Another form of amnesia is anterograde amnesia—the inability to form new memories. Anterograde means “forward moving.” The most famous case study of anterograde amnesia lasted over 50 years. It was of a man who for years was known only by his initials—H.M. But the need to protect H.M.’s privacy ended when Henry Molaison died at the age of 82 on December 2, 2008.
anterograde amnesia
Loss of memory caused by the inability to store new memories; forward-acting amnesia.
In 1953, Henry was 27 years old and had a 10-year history of severe, untreatable epileptic seizures. Henry’s doctors located the brain area where the seizures seemed to originate. With no other options available at the time, the decision was made to surgically remove portions of the medial (inner) temporal lobe on each side of Henry’s brain, including the brain structure called the hippocampus (Scoville & Milner, 1957). Portions of the left and right amygdala were also removed (Annese & others, 2014).
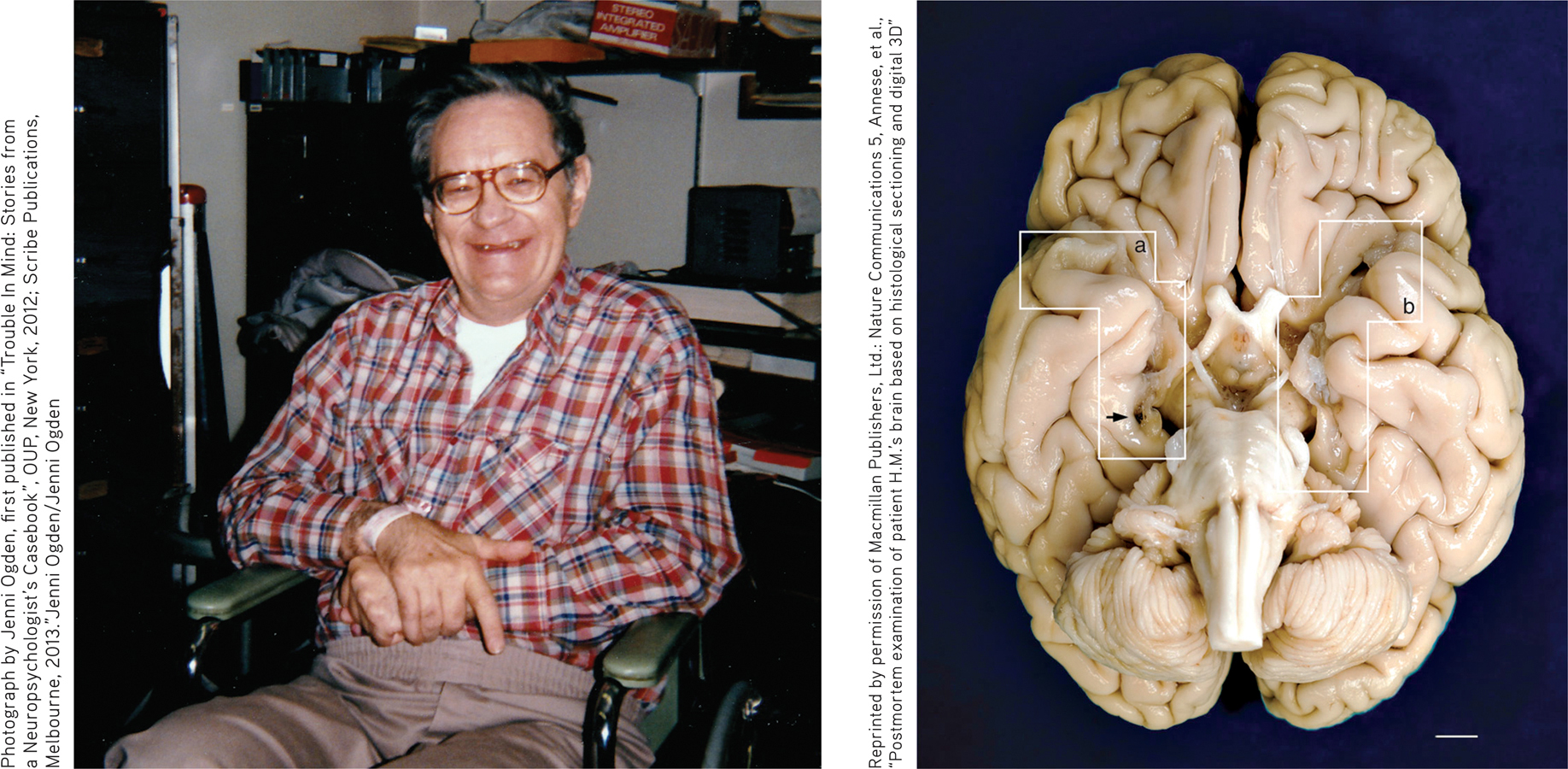
Because of the profound anterograde amnesia caused by the surgery, Henry became one of the most intensive case studies in psychology and neuroscience. Over the next half century, Henry participated in hundreds of studies that fundamentally altered the scientific understanding of memory.
Henry died on December 2, 2008, but his contributions to science continue. Neuroscientist Jacopo Annese and his team (2014) dissected Henry’s brain, slicing it into 2,400 micro-thin slices in a marathon, 3-day session that was streamed online. Photographs of each slice were taken to create a virtual 3-D digital model of Henry’s brain. The white boxes (a) and (b) show where portions of the hippocampus and amygdala on the left and right side of Henry’s brain were removed. The researchers also discovered a previously unknown small lesion on Henry’s left frontal lobe, which they speculate is probably scar tissue from the original surgery. You can watch a short video of the dissection at http:/
Reprinted by permission of Macmillan Publishers, Ltd.: Nature Communications 5, Annese, et al., “Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D”
After the experimental surgery, the frequency and severity of Henry’s seizures were greatly reduced. However, it was quickly discovered that Henry’s ability to form new memories of events and information had been destroyed. Although the experimental surgery had treated H.M.’s seizures, it also dramatically revealed the role of the hippocampus in forming new explicit memories for episodic and semantic information.
Psychologists Brenda Milner (1970) and Suzanne Corkin in (2013) studied Henry extensively over the past 50 years. If you had had the chance to meet Henry, he would have appeared normal enough. He had a good vocabulary and social skills, normal intelligence, and a delightful sense of humor. And he was well aware of his memory problem. When Suzanne Corkin (2002) once asked him, “What do you do to try to remember?” Henry quipped, “Well, that I don’t know because I don’t n’t remember [chuckle] what I tried.”
Despite superficially appearing normal, Henry lived in the eternal present. Had you talked with Henry for 15 minutes, then left the room for 2 or 3 minutes before coming back, he wouldn’t remember having met you before. Although some of the psychologists and doctors had treated Henry for years, even decades, Henry was meetinging them for the first time on each occasion he interacted with them (Corkin, 2013; MacKay, 2014).

For the most part, Henry’s short-term memory worked just fine. In fact, he could fool you. If Henry actively repeated or rehearsed information, he could hold it in short-term memory for an hour or more (Nader & Wang, 2006). Yet just moments after he switched his attention to something else and stopped rehearsing the information, it was gone forever. However, Henry’s long-term memory was partially intact. He could retrieve long-term memories from before the time he was 16 years old, when the severe epileptic seizures began.
In general, Henry was unable to acquire new long-term memories of events (episodic information) or general knowledge (semantic information). Still, every now and then, Henry surprised his doctors and visitors with some bit of knowledge that he acquired after the surgery (see the In Focus box “H.M. and Famous People”).
Henry’s case suggests that the hippocampus is not involved in most short-term memory tasks, nor is it the storage site for already established long-term memories. Instead, the critical role played by the hippocampus seems to be the encoding of new memories for events and information and the transfer of those new memories from short-term to long-term memory.
Implicit and Explicit Memory in Anterograde AmnesiaHenry’s case and those of other patients with anterograde amnesia have contributed greatly to our understanding of implicit versus explicit memories. To refresh your memory, implicit memories are memories without conscious awareness. In contrast, explicit memories are memories with conscious awareness.
IN FOCUS
H.M. and Famous People



When his hippocampus was removed, Henry Molaison lost the ability to quickly encode new semantic and episodic memories. For example, he was unable to learn new vocabulary words or remember people he had met. But is the hippocampus necessary for all semantic learning? Or is it possible that other brain areas might support some limited learning of new knowledge?
To test this idea, psychologists Gail O’Kane, Elizabeth Kensinger, and Suzanne Corkin (2004) evaluated Henry for his knowledge of people who became famous after his surgery in 1953. On the first day, Henry was given the famous person’s first name as a cue and asked to say the last name that came to his mind. Examples were “Elvis ____________ (Presley)” and “Fidel ____________ (Castro),” who first became famous during the 1950s; “Lyndon ______________ (Johnson)” and “Ray ______________ (Charles)” from the 1960s; “Sophia ______________ (Loren)” from the 1970s; and “Ronald ______________ (Reagan)” from the 1980s. Henry was able to correctly supply the last name of 12 out of 35 famous people, including Martin Luther King, Sophia Loren, and Ronald Reagan.
In a second test on the next day, Henry was able to generate the last names for an additional 11 famous people after being given background information about them. For example, provided with the details “famous artist, born in Spain, formulated Cubism, works include Guernica,” Henry responded “Picasso” to the cue “first name is Pablo.”
Henry’s ability to generate the last names of well-known people indicated that he had acquired some declarative semantic knowledge. O’Kane and her colleagues (2004) wondered whether Henry could go beyond this superficial knowledge and provide specific details. In a different test, Henry was able to provide two or more pieces of information about 12 people who had become prominent after the onset of his amnesia.
For example, after correctly identifying John F. Kennedy as a famous person, Henry indicated that Kennedy was Catholic, had become president, that somebody shot him, and that he didn’t survive. Henry was also able to provide details about Woody Allen, astronaut John Glenn, musician Ray Charles, and film star Sophia Loren.
According to O’Kane and her colleagues (2004), “These results provide robust, unambiguous evidence that at least some semantic learning can be supported by structures beyond the hippocampus.” However, the limitations of Henry’s semantic learning must also be stressed. Henry was still unable to quickly acquire new semantic or episodic memories. It was only after years of extended repetitions of information that Henry acquired some limited bits and pieces of new knowledge about a few famous people.
Henry could not form new episodic or semantic memories, which reflects the explicit memory system. But he could form new procedural memories, which reflects the implicit memory system. For example, when given the same logical puzzle to solve several days in a row, Henry was able to solve it more quickly each day. This improvement showed that he implicitly “remembered” the procedure involved in solving the puzzle. But if you asked Henry if he had ever seen the puzzle before, he would answer “no” because he could not consciously (or explicitly) remember having learned how to solve the puzzle. This suggests that the hippocampus is less crucial to the formation of new implicit memories, such as procedural memories, than it is to the formation of new explicit memories.
Were Henry’s memory anomalies an exception? Not at all. Studies conducted with other people who have sustained damage to the hippocampus and related brain structures showed the same anterograde amnesia (see Bayley & Squire, 2002). Like Henry, these patients are unable to form new explicit memories, but their performances on implicit memory tasks, which do not require conscious recollection of the new information, are much closer to normal. Such findings indicate that implicit and explicit memory processes involve different brain structures and pathways.
BRAIN STRUCTURES INVOLVED IN MEMORY
Along with the hippocampus, several other brain regions involved in memory include the cerebellum, the amygdala, and the frontal cortex (see FIGURE 6.11). As you saw earlier, the cerebellum is involved in classically conditioning simple reflexes, such as the eye-blink reflex. The cerebellum is also involved in procedural memories and other motor skill memories.
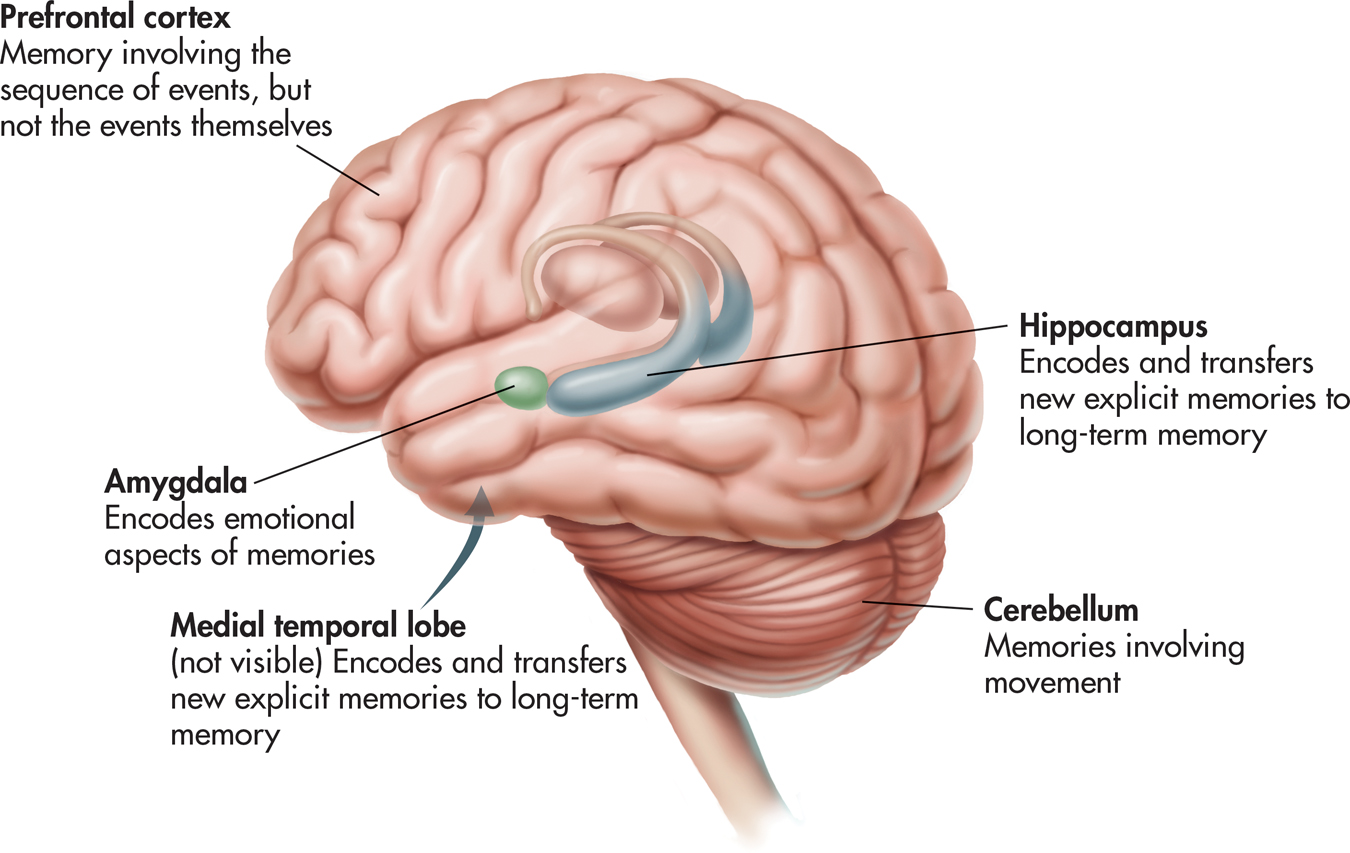
The amygdala, which is situated very close to the hippocampus, is involved in encoding and storing the emotional qualities associated with particular memories, such as fear or anger (Hamann, 2009; McGaugh, 2004). For example, normal monkeys are afraid of snakes. But if the amygdala is damaged, a monkey loses its fear of snakes and other natural predators.
The frontal lobes are involved in retrieving and organizing information that is associated with autobiographical and episodic memories (Greenberg & Rubin, 2003). The prefrontal cortex seems to play an important role in working memory (McNab & Klingberg, 2008).
The medial temporal lobes, like the frontal lobes, do not actually store the information that comprises our autobiographical memories. Rather, they are involved in encoding complex memories, by forming links among the information stored in multiple brain regions (Greenberg & Rubin, 2003; Shrager & Squire, 2009). As we described in the Focus on Neuroscience, “Assembling Memories,” on page 258, retrieving a memory activates the same brain regions that were involved in initially encoding the memory.
ALZHEIMER’S DISEASEGRADUALLY LOSING THE ABILITY TO REMEMBER

For example, the outward signs of Alzheimer’s disease (AD) and the degree of brain damage evident at death are not perfectly correlated. Although some nuns had clear brain evidence of AD, they did not display observable cognitive and behavior declines prior to their deaths. Other nuns had only mild brain evidence of AD but showed severe cognitive and behavioral declines.
Interestingly, the sisters who displayed better language abilities when they were young women were less likely to display AD symptoms. This held true regardless of how much brain damage was evident at the time of their death (Iacono & others, 2009). As researcher Diego Iacono (2009) commented, “It’s the first time that we’re shown that a complex cognitive activity, like language ability, is connected with a neurodegenerative disease.”
Understanding how the brain processes and stores memories has important implications. Dementia is a broad term that refers to the decline and impairment of memory, reasoning, language, and other cognitive functions. These cognitive disruptions occur to such an extent that they interfere with the person’s ability to carry out daily activities. Dementia is not a disease itself. Rather, it describes a group of symptoms that often accompany a disease or a condition.
dementia
Progressive deterioration and impairment of memory, reasoning, and other cognitive functions as the result of disease, injury, or substance abuse.
The most common cause of dementia is Alzheimer’s disease (AD). It is estimated that about 5.4 million Americans suffer from AD. That number is expected to dramatically escalate as more and more “baby boomers” reach age 65. The disease usually doesn’t begin until after age 60, but the risk goes up with age. About 6 percent of men and women in the 65–
Alzheimer’s disease (AD)
A progressive disease that destroys the brain’s neurons, gradually impairing memory, thinking, language, and other cognitive functions, resulting in the complete inability to care for oneself; the most common cause of dementia.
Although the cause or causes of Alzheimer’s disease are still unknown, it is known that the brains of AD patients develop an abundance of two abnormal structures—beta-amyloid plaques and neurofibrillary tangles (Ballard & others, 2011). The plaques are dense deposits of protein and other cell materials outside and around neurons. The plaques interfere with the ability of neurons to communicate, damaging the neurons to the point that they die. The tangles are twisted fibers that build up inside the neuron and interrupt the flow of nourishment to the neuron, ultimately causing the neuron to die. Although most older people develop some plaques and tangles in their brains, the brains of AD patients have them to a much greater extent (Petersen, 2002). The Focus on Neuroscience vividly portrays the progressive loss of neurons that is the root cause of Alzheimer’s disease.
FOCUS ON NEUROSCIENCE
Mapping Brain Changes in Alzheimer’s Disease
The hallmark of Alzheimer’s disease is its relentless, progressive destruction of neurons in the brain, turning once-healthy tissue into a tangled, atrophied mass. This progressive loss of brain tissue is dramatically revealed in the MRI images shown below. Created by neuroscientist Paul Thompson and his colleagues (2003), these high-resolution “brain maps” represent composite images of the progressive effects of Alzheimer’s disease (AD) in 12 patients over the course of two years. In these color-coded images, blue corresponds to normal tissue (no loss), red indicates up to 10 percent tissue loss, and white indicates up to 20 percent tissue loss.
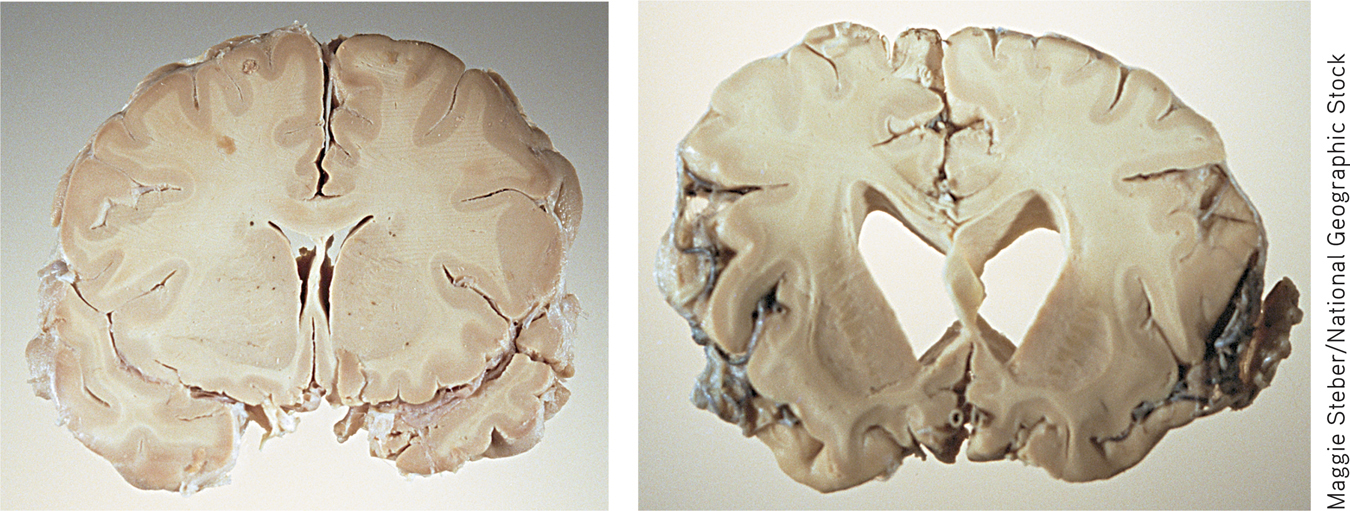
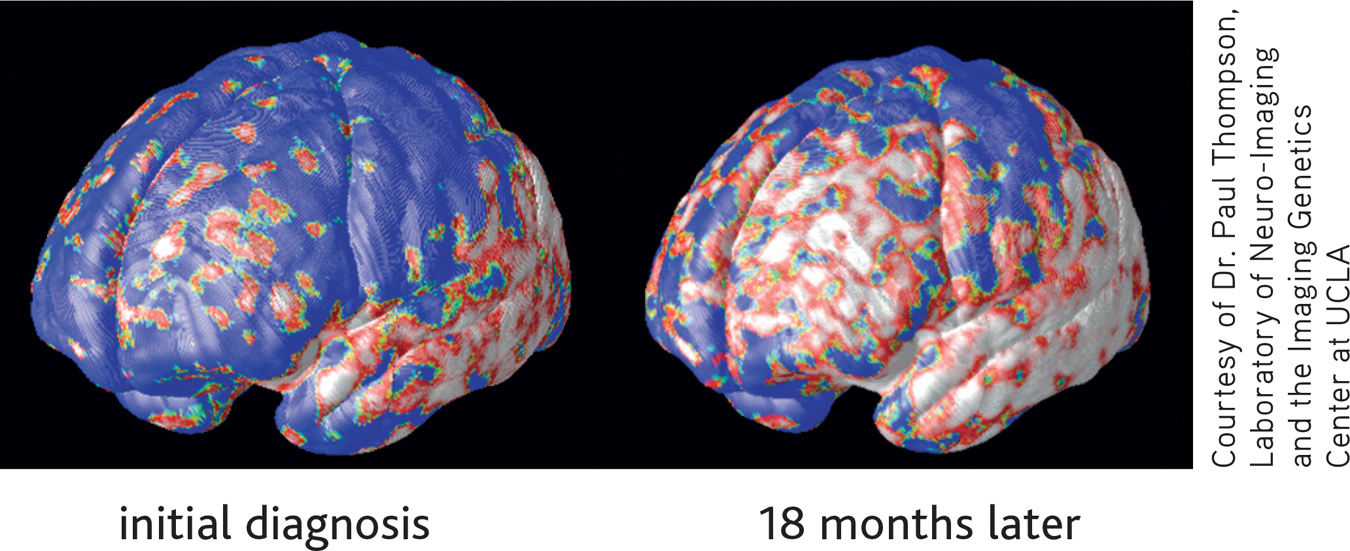
Thompson likens the progression of AD to that of molten lava flowing around rocks—the disease leaves islands of brain tissue unscathed. The disease first attacks the temporal lobes, affecting areas involved in memory, especially short-term memory. Next affected are the frontal areas, which are involved in thinking, reasoning, self-control, and planning ahead. You can also see significant internal loss in limbic areas, which are involved in regulating emotion. At this point in the progression of AD, there is very little loss in sensory and visual brain areas. Eventually the disease engulfs the entire brain. The photos above contrast cross sections of a normal brain (left) and the brain of a person who died of Alzheimer’s disease (right). In the normal brain, the temporal lobes are intact. The ventricles, which hold the cerebral spinal fluid, are slender. In the brain ravaged by Alzheimer’s, the gaping ventricles extend into the space left by the death of brain cells in the temporal lobes.
In the early stages of AD, the symptoms of memory impairment are often mild, such as forgetting the names of familiar people, forgetting the location of familiar places, or forgetting to do things. But as the disease progresses, memory loss and confusion become more pervasive. The person becomes unable to remember what month it is or the names of family members. Frustrated and disoriented by the inability to retrieve even simple information, the person can become agitated and moody. In the last stage of AD, internal brain damage has become widespread. The person no longer recognizes loved ones and is unable to communicate in any meaningful way. All sense of self and identity has vanished. At the closing stages, the person becomes completely incapacitated. Ultimately, Alzheimer’s disease is fatal (Alzheimer’s Association, 2011).
Some 14.9 million Americans provide unpaid care for a person with Alzheimer’s disease or other dementia. These unpaid caregivers are primarily family members but also include friends and neighbors. In 2010, these caregivers provided 17 billion hours of unpaid care (Alzheimer’s Association, 2011). Not only is there a financial toll, but families and caregivers struggle with great physical and emotional stress as they try to cope with the mental and physical changes occurring in their loved one. The average number of hours of unpaid care provided for a relative or friend with Alzheimer’s increases as the person’s condition worsens.
Numerous resources, such as the Alzheimer’s Disease Education & Referral Center (www.alzheimers.org) and the Alzheimer’s Association (www.alz.org), are available to help support families and other caregivers.
Test your understanding of Biological Basis of Memory with
 .
.
Closing Thoughts
Human memory is at once both perfectly ordinary and quite extraordinary. With next to no mental effort, you form and recall countless memories as you go through daily life. Psychologists have made enormous progress in explaining how those memories are encoded, stored, retrieved, and forgotten.
Perhaps the most fascinating aspect of human memory is its fallibility. Memory is surprisingly susceptible to errors and distortions. Under some conditions, completely false memories can be experienced, such as Elizabeth Loftus’s memory of discovering her mother’s body in the swimming pool. Such false memories can be so subjectively compelling that they feel like authentic memories, yet confidence in a memory is not proof of the memory’s truth.
Many mysteries of human memory remain, including exactly how memories are stored in and retrieved from the brain. Nevertheless, reliable ways of improving memory in everyday life have been discovered. In the Psych for Your Life feature, we provide several suggestions to enhance your memory for new information.