5.4 Bacteriophage Genetics
The word bacteriophage, which is a name for bacterial viruses, means “eater of bacteria.” These viruses parasitize and kill bacteria. Pioneering work on the genetics of bacteriophages in the middle of the twentieth century formed the foundation of more recent research on tumor-
These viruses can be used in two different types of genetic analysis. First, two distinct phage genotypes can be crossed to measure recombination and hence map the viral genome. Mapping of the viral genome by this method is the topic of this section. Second, bacteriophages can be used as a way of bringing bacterial genes together for linkage and other genetic studies. We will study the use of phages in bacterial studies in Section 5.5. In addition, as we will see in Chapter 10, phages are used in DNA technology as carriers, or vectors, of foreign DNA. Before we can understand phage genetics, we must first examine the infection cycle of phages.
Infection of bacteria by phages
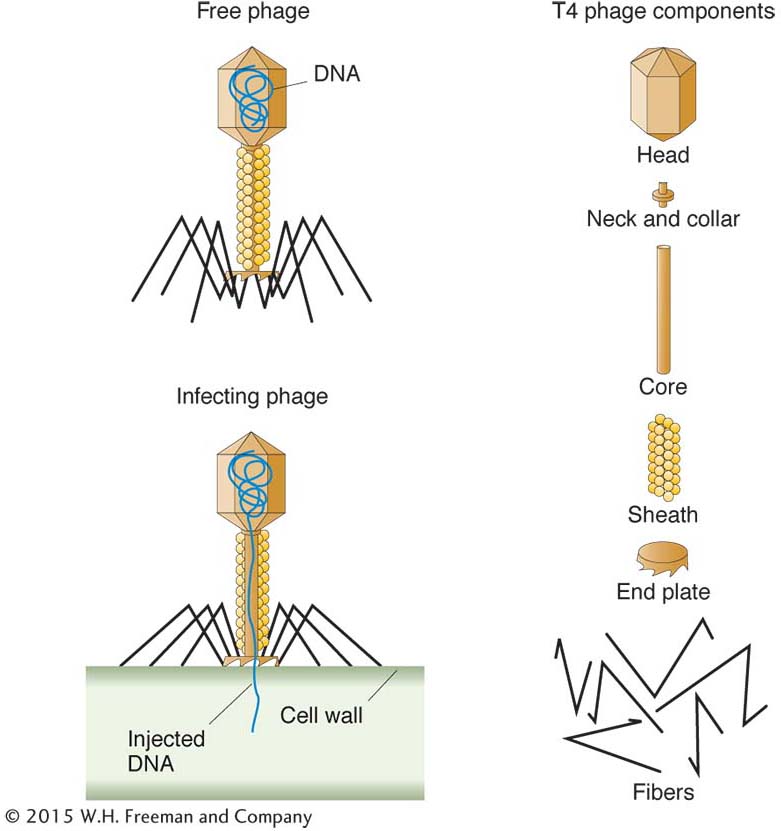
Most bacteria are susceptible to attack by bacteriophages. A phage consists of a nucleic acid “chromosome” (DNA or RNA) surrounded by a coat of protein molecules. Phage types are identified not by species names but by symbols—
How can we study inheritance in phages when they are so small that they are visible only under the electron microscope? In this case, we cannot produce a visible colony by plating, but we can produce a visible manifestation of a phage by taking advantage of several phage characters.
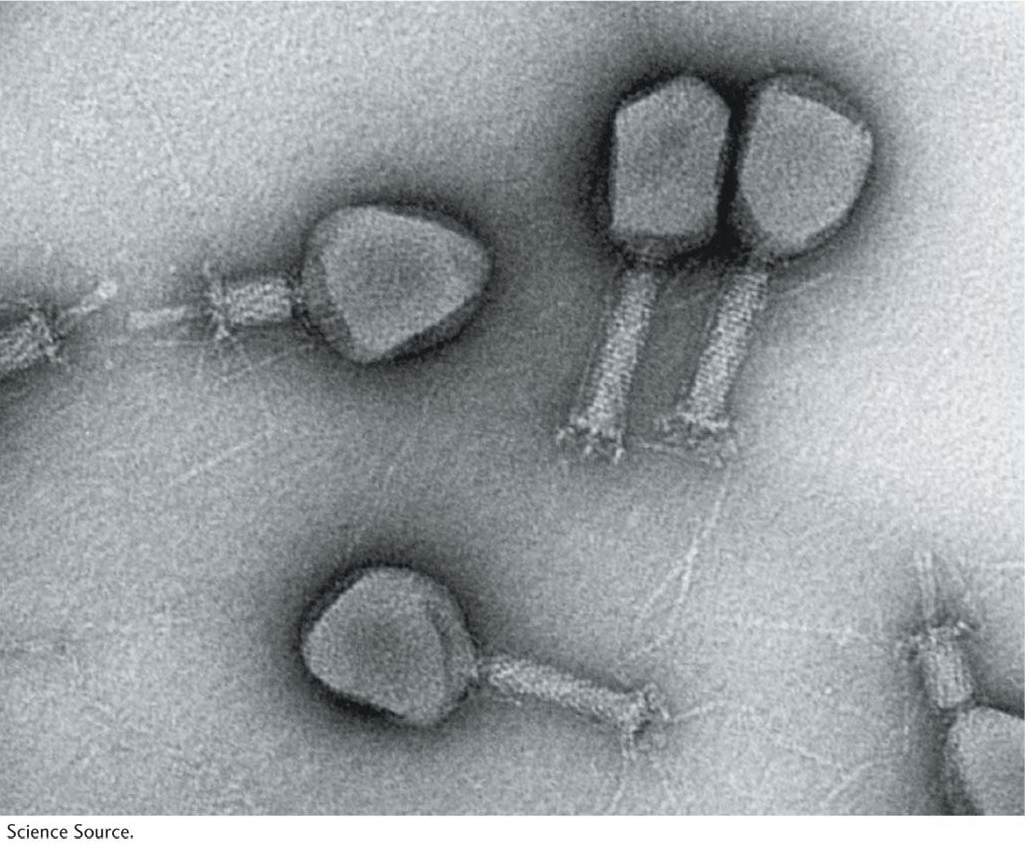
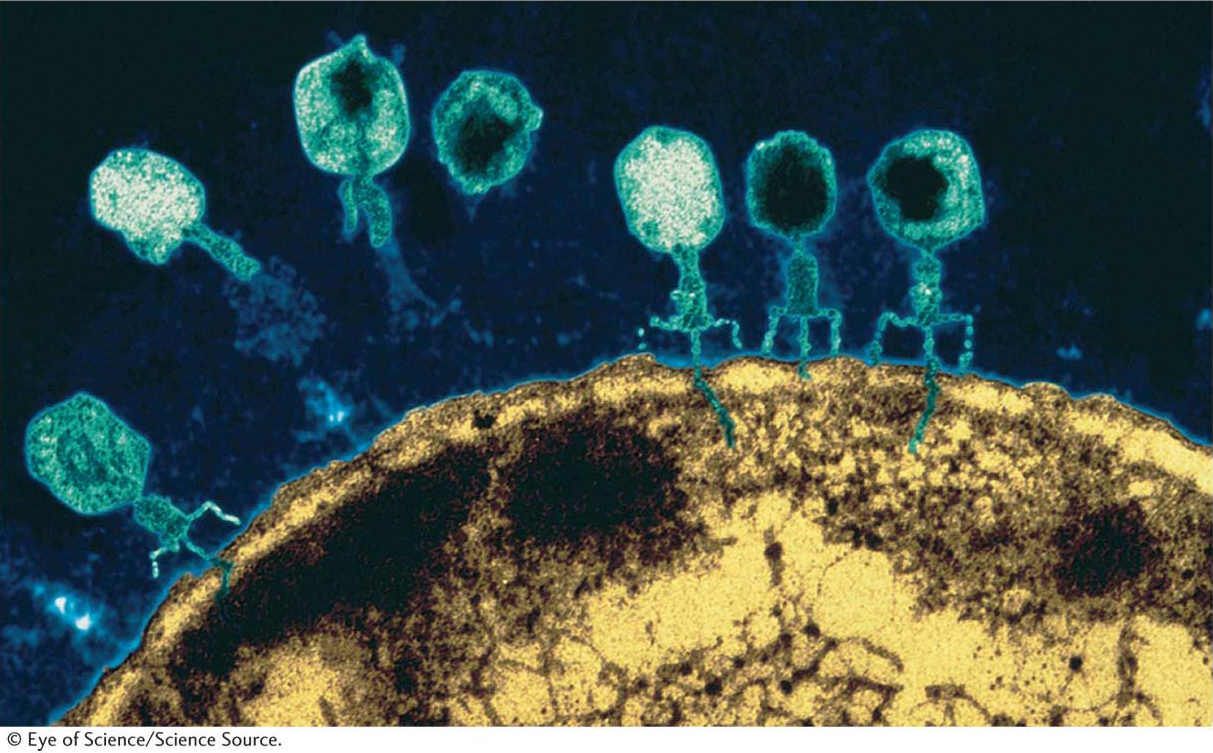
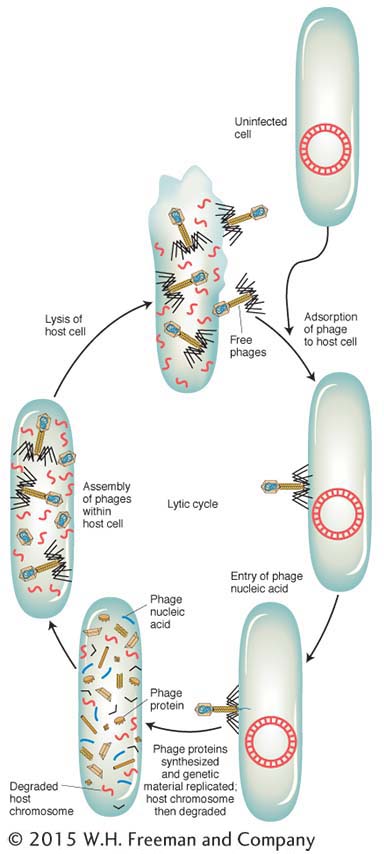
Let’s look at the consequences of a phage infecting a single bacterial cell. Figure 5-25 shows the sequence of events in the infectious cycle that leads to the release of progeny phages from the lysed cell. After lysis, the progeny phages infect neighboring bacteria. This cycle is repeated through progressive rounds of infection, and, as these cycles repeat, the number of lysed cells increases exponentially. Within 15 hours after one single phage particle infects a single bacterial cell, the effects are visible to the naked eye as a clear area, or plaque, in the opaque lawn of bacteria covering the surface of a plate of solid medium (Figure 5-26). Such plaques can be large or small, fuzzy or sharp, and so forth, depending on the phage genotype. Thus, plaque morphology is a phage character that can be analyzed at the genetic level. Another phage phenotype that we can analyze genetically is host range, because phages may differ in the spectra of bacterial strains that they can infect and lyse. For example, a specific strain of bacteria might be immune to phage 1 but susceptible to phage 2.

Mapping phage chromosomes by using phage crosses
Two phage genotypes can be crossed in much the same way that we cross organisms. A phage cross can be illustrated by a cross of T2 phages originally studied by Alfred Hershey. The genotypes of the two parental strains in Hershey’s cross were h− r+ × h+ r−. The alleles correspond to the following phenotypes:
h− : can infect two different E. coli strains (which we can call strains 1 and 2)
h+ : can infect only strain 1
r− : rapidly lyses cells, thereby producing large plaques
r+ : slowly lyses cells, producing small plaques
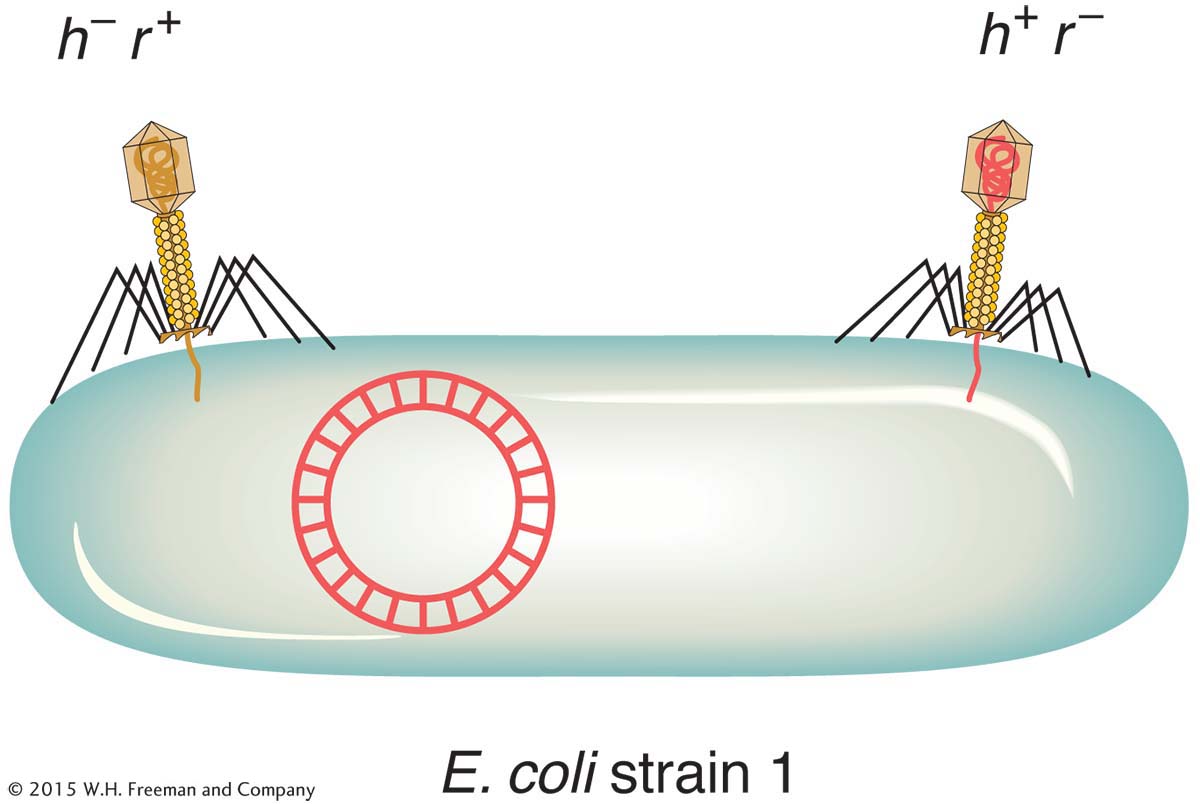
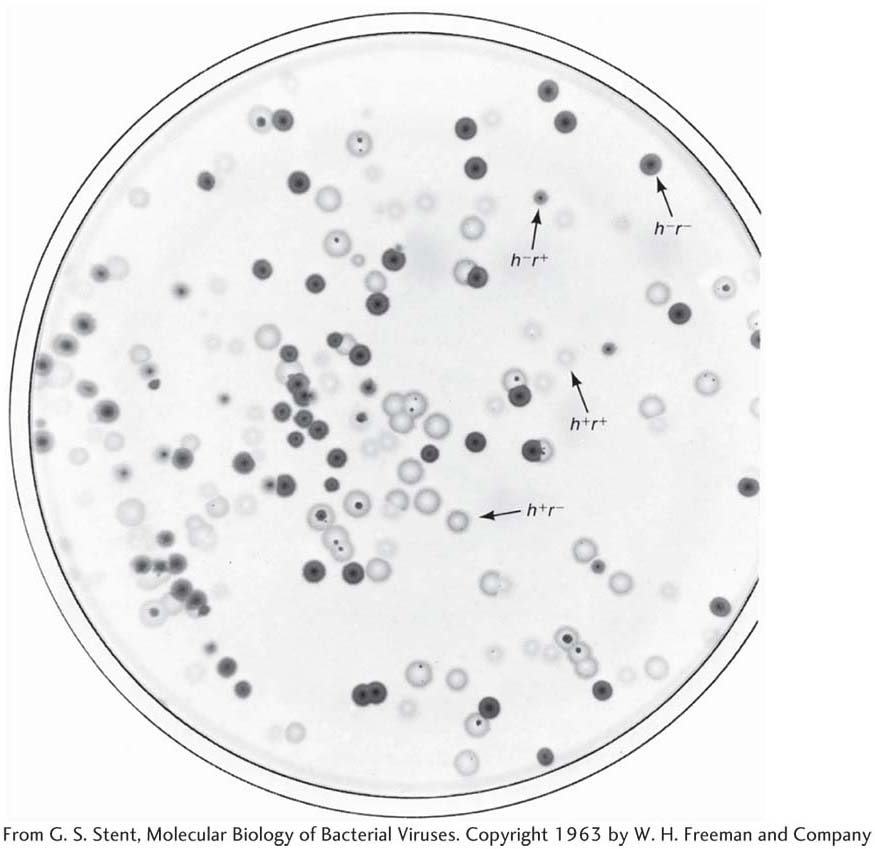
To make the cross, E. coli strain 1 is infected with both parental T2 phage genotypes. This kind of infection is called a mixed infection or a double infection (Figure 5-27). After an appropriate incubation period, the phage lysate (containing the progeny phages) is analyzed by spreading it onto a bacterial lawn composed of a mixture of E. coli strains 1 and 2. Four plaque types are then distinguishable (Figure 5-28). Large plaques indicate rapid lysis (r−), and small plaques indicate slow lysis (r+). Phage plaques with the allele h− will infect both hosts, forming a clear plaque, whereas phage plaques with the allele h+ will infect only one host, forming a cloudy plaque. Thus, the four genotypes can be easily classified as parental (h− r+ and h+ r−) and recombinant (h+ r+ and h− r−), and a recombinant frequency can be calculated as follows:
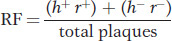
If we assume that the recombining phage chromosomes are linear, then single crossovers produce viable reciprocal products. However, phage crosses are subject to some analytical complications. First, several rounds of exchange can take place within the host: a recombinant produced shortly after infection may undergo further recombination in the same cell or in later infection cycles. Second, recombination can take place between genetically similar phages as well as between different types. Thus, if we let P1 and P2 refer to general parental genotypes, crosses of P1 × P1 and P2 × P2 take place in addition to P1 × P2. For both these reasons, recombinants from phage crosses are a consequence of a population of events rather than defined, single-
Because astronomically large numbers of phages can be used in phage-
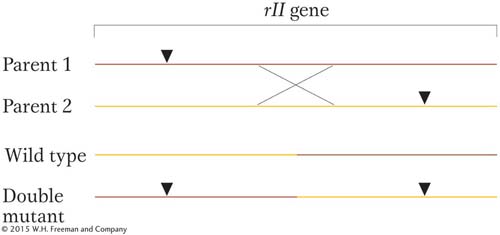
As distance between two mutant sites increases, such a crossover event is more likely. Thus, the frequency of rII+ recombinants is a measure of that distance within the gene. (The reciprocal product is a double mutant and indistinguishable from the parentals.)
Benzer used a clever approach to detect the very rare rII+ recombinants. He made use of the fact that rII mutants will not infect a strain of E. coli called K. Therefore, he made the rII × rII cross on another strain and then plated the phage lysate on a lawn of strain K. Only rII+ recombinants will form plaques on this lawn. This way of finding a rare genetic event (in this case, a recombinant) is a selective system: only the desired rare event can produce a certain visible outcome. In contrast, a screen is a system in which large numbers of individuals are visually scanned to seek the rare “needle in the haystack.”
This same approach can be used to map mutant sites within genes for any organism from which large numbers of cells can be obtained and for which wild-