6.1 Interactions Between the Alleles of a Single Gene: Variations on Dominance
There are thousands of different ways to alter the sequence of a gene, each producing a mutant allele, although only some of these mutant alleles will appear in a real population. The known mutant alleles of a gene and its wild-
One of the tests routinely performed on a new mutant allele is to see if it is dominant or recessive. Basic information about dominance and recessiveness is useful in working with the new mutation and can be a source of insight into the way the gene functions, as we will see in the examples. Dominance is a manifestation of how the alleles of a single gene interact in a heterozygote. In any experiment the interacting alleles may be wild type and mutant alleles (+/m) or two different mutant alleles (m1/m2). Several types of dominance have been discovered, each representing a different type of interaction between alleles.
Complete dominance and recessiveness
The simplest type of dominance is full, or complete, dominance, which we examined in Chapter 2. A fully dominant allele will be expressed in the phenotype when only one copy is present, as in a heterozygote, whereas the alternative allele will be fully recessive. In full dominance, the homozygous dominant cannot be distinguished from the heterozygote; that is, at the phenotypic level, A/A = A/a. As mentioned earlier, phenylketonuria (PKU) and many other single-
217
The disease PKU is a good general model for recessive mutations. Recall that PKU is caused by a defective allele of the gene encoding the enzyme phenylalanine hydroxylase (PAH). In the absence of normal PAH, the phenylalanine entering the body in food is not broken down and hence accumulates. Under such conditions, phenylalanine is converted into phenylpyruvic acid, which is transported to the brain through the bloodstream and there impedes normal development, leading to mental retardation. The reason that the defective allele is recessive is that one “dose” of the wild-
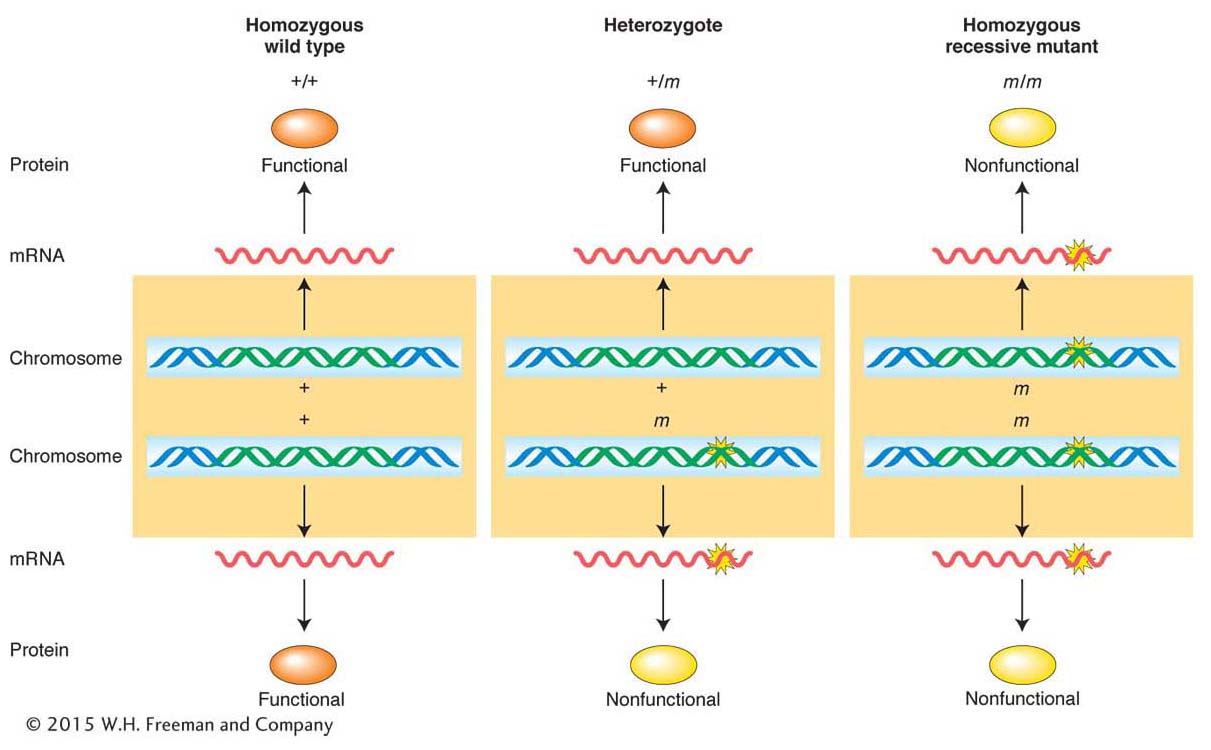
How can we explain fully dominant mutations? There are several molecular mechanisms for dominance. A regularly encountered mechanism is that the wild-
218
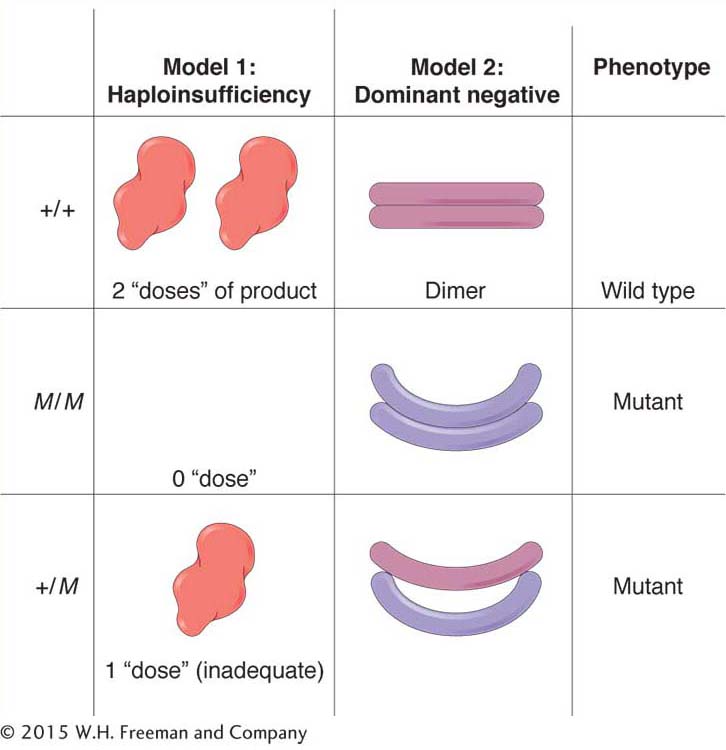
Another important type of dominant mutation is called a dominant negative. Polypeptides with this type of mutation act as “spoilers” or “rogues.” In some cases, the gene product is a unit of a homodimeric protein, a protein composed of two units of the same type. In the heterozygote (+/M), the mutant polypeptide binds to the wild-
An example of mutations that can act as dominant negatives is found in the gene for collagen protein. Some mutations in this gene give rise to the human phenotype osteogenesis imperfecta (brittle-
KEY CONCEPT
For most genes, a single wild-Incomplete dominance
Four-
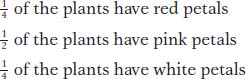
219

ANIMATED ART: Molecular allele interactions
Figure 6-3 shows these phenotypes. From this 1:2:1 ratio in the F2, we can deduce that the inheritance pattern is based on two alleles of a single gene. However, the heterozygotes (the F1 and half the F2) are intermediate in phenotype. By inventing allele symbols, we can list the genotypes of the four-
How do we explain incomplete dominance at the molecular level? In incomplete dominance, each wild-
Codominance
Another variation on the theme of dominance is codominance, the expression of both alleles of a heterozygote. A clear example is seen in the human ABO blood groups, where there is codominance of antigen alleles. The ABO blood groups are determined by three alleles of one gene. These three alleles interact in several ways to produce the four blood types of the ABO system. The three major alleles are i, IA, and IB, but a person can have only two of the three alleles or two copies of one of them. The combinations result in six different genotypes: the three homozygotes and three different types of heterozygotes, as follows.
|
Genotype |
Blood type |
|---|---|
|
IA/IA, IA/i |
A |
|
IB/IB, IB/i |
B |
|
IA/IB |
AB |
|
i/i |
O |
In this allelic series, the alleles determine the presence and form of a complex sugar molecule present on the surface of red blood cells. This sugar molecule is an antigen, a cell-
The human disease sickle-
220
|
HbA/HbA: |
normal; red blood cells never sickle |
|
|
HbS/HbS: |
severe, often fatal anemia; abnormal hemoglobin causes red blood cells to have sickle shape |
|
|
HbA/HbS: |
no anemia; red blood cells sickle only under low oxygen concentrations |
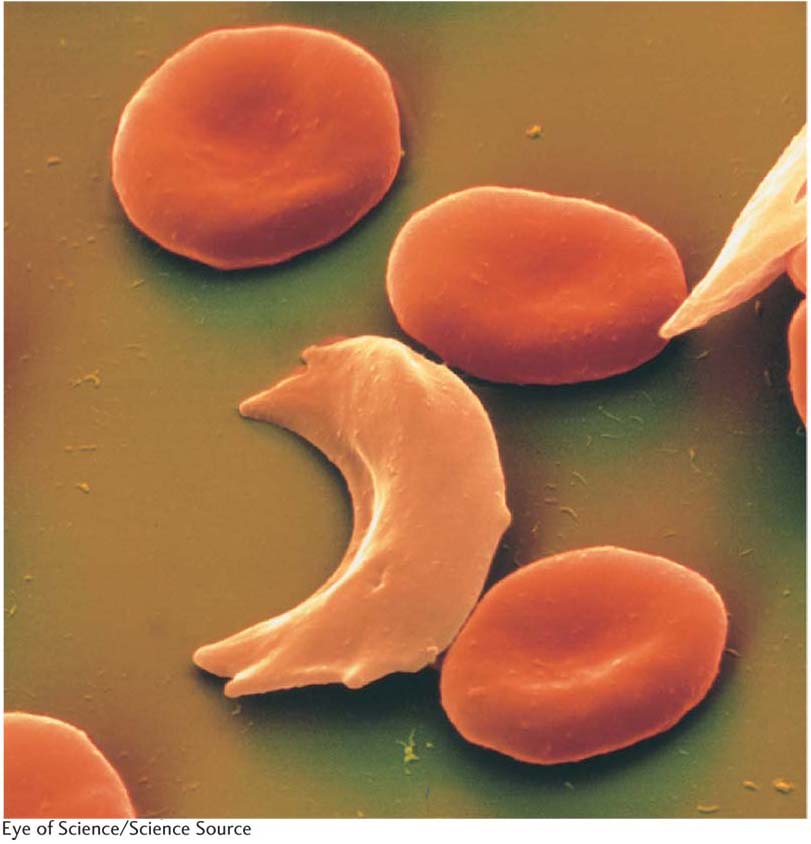
Figure 6-4 shows an electron micrograph of blood cells including some sickled cells. In regard to the presence or absence of anemia, the HbA allele is dominant. In the heterozygote, a single HbA allele produces enough functioning hemoglobin to prevent anemia. In regard to blood-
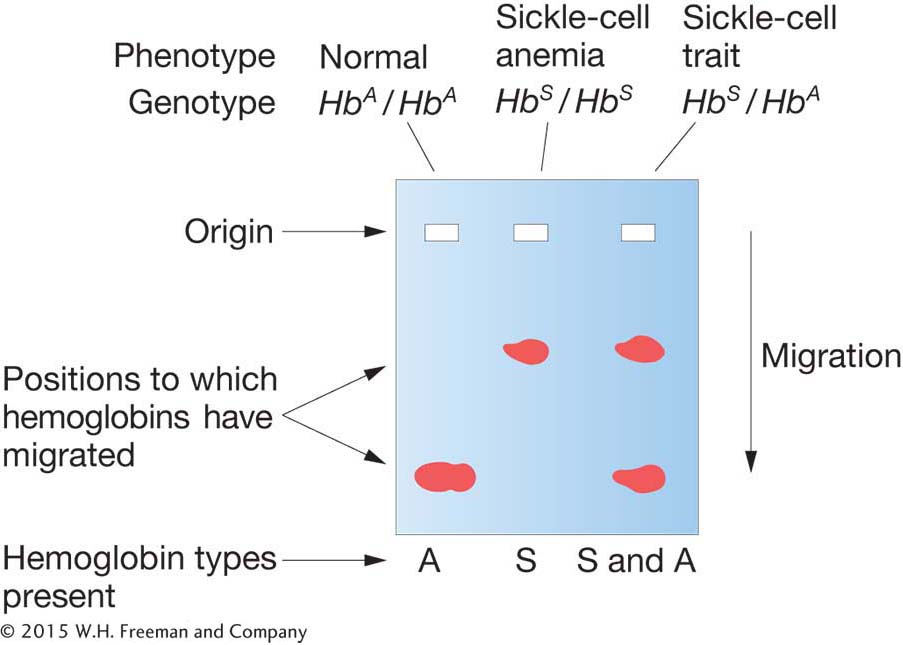
Sickle-
KEY CONCEPT
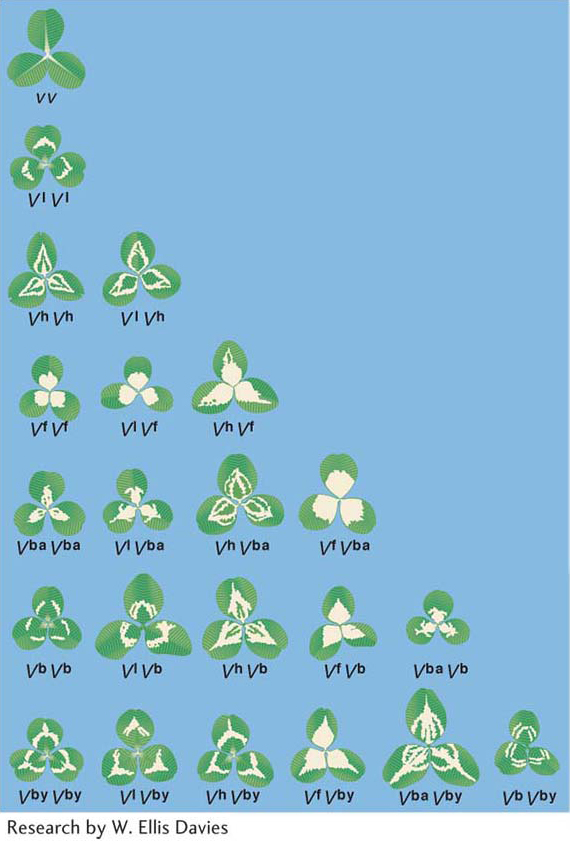
In general, three main types of dominance can be distinguished: full dominance, incomplete dominance, and codominance. The type of dominance is determined by the molecular functions of the alleles of a gene and by the investigative level of analysis.The leaves of clover plants show several variations on the dominance theme. Clover is the common name for plants of the genus Trifolium. There are many species. Some are native to North America, whereas others grow there as introduced weeds. Much genetic research has been done with white clover, which shows considerable variation among individual plants in the curious V, or chevron, pattern on the leaves. The different chevron forms (and the absence of chevrons) are determined by a series of seven alleles, as seen in Figure 6-6, which shows the many different types of interactions possible for even one allele. In most practical cases many alleles of a gene can be found together in a population, constituting an allelic series. The phenotypes shown by the allelic combinations are many and varied, reflecting the relative nature of dominance: an allele can show dominance with one partner but not with another. Hence, the complexity illustrated by the ABO blood type system is small compared with that in a case such as clover chevrons.
Recessive lethal alleles
An allele that is capable of causing the death of an organism is called a lethal allele. In the characterization of a set of newly discovered mutant alleles, a recessive mutation is sometimes found to be lethal. This information is potentially useful in that it shows that the newly discovered gene (of yet unknown function) is essential to the organism’s operation. Essential genes are those without which an organism dies. (An example of an essential gene might be a ribosomal gene without which no protein would be made.) Indeed, with the use of modern DNA technology, a null mutant allele of a gene of interest can now be made intentionally and made homozygous to see if it is lethal and under which environmental conditions. Lethal alleles are also useful in determining the developmental stage at which the gene normally acts. In this case, geneticists look for whether death from a lethal mutant allele occurs early or late in the development of a zygote. The phenotype associated with death can also be informative in regard to gene function; for example, if a certain organ appears to be abnormal, the gene is likely to be expressed in that organ.
221
What is the diagnostic test for lethality? The test is well illustrated by one of the prototypic examples of a lethal allele, a coat-
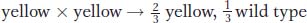
Figure 6-7 shows a typical litter from a cross between yellow mice.

How can the 2:1 ratio be explained? The results make sense if the yellow allele is assumed to be lethal when homozygous. The yellow allele is known to be of a coat-
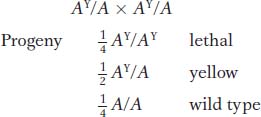
The expected monohybrid ratio of 1 :2 :1 would be found among the zygotes, but it is altered to a 2 :1 ratio in the progeny actually seen at birth because zygotes with a lethal AY/AY genotype do not survive to be counted. This hypothesis is supported by the removal of uteri from pregnant females of the yellow × yellow cross; one-
The AY allele produces effects on two characters: coat color and survival. It is entirely possible, however, that both effects of the AY allele result from the same basic cause, which promotes yellowness of coat in a single dose and death in a double dose. In general, the term pleiotropic is used for any allele that affects several properties of an organism.
222
Mouse
The laboratory mouse is descended from the house mouse Mus musculus. The pure lines used today as standards are derived from mice bred in past centuries by mouse “fanciers.” Among model organisms, it is the one whose genome most closely resembles the human genome. Its diploid chromosome number is 40 (compared with 46 in humans), and the genome is slightly smaller than that of humans (the human genome being 3000 Mb) and contains approximately the same number of genes (current estimate 25,000). Furthermore, all mouse genes seem to have counterparts in humans. A large proportion of genes are arranged in blocks in exactly the same positions as those of humans.
Research on the Mendelian genetics of mice began early in the twentieth century. One of the most important early contributions was the elucidation of the genes that control coat color and pattern. Genetic control of the mouse coat has provided a model for all mammals, including cats, dogs, horses, and cattle. A great deal of work was also done on mutations induced by radiation and chemicals. Mouse genetics has been of great significance in medicine. A large proportion of human genetic diseases have mouse counterparts useful for experimental study (they are called “mouse models”). The mouse has played a particularly important role in the development of our current understanding of the genes underlying cancer.
The mouse genome can be modified by the insertion of specific fragments of DNA into a fertilized egg or into somatic cells. The mice in the photograph have received a jellyfish gene for green fluorescent protein (GFP) that makes them glow green under special lights. Gene knockouts and replacements also are possible.
A major limitation of mouse genetics is its cost. Whereas working with a million individuals of E. coli or S. cerevisiae is a trivial matter, working with a million mice requires a factory-

The tailless Manx phenotype in cats (Figure 6-8) also is produced by an allele that is lethal in the homozygous state. A single dose of the Manx allele, ML, severely interferes with normal spinal development, resulting in the absence of a tail in the ML/M heterozygote. But in the ML/ML homozygote, the double dose of the gene produces such an extreme abnormality in spinal development that the embryo does not survive.

The yellow and ML alleles have their own phenotypes in a heterozygote, but most recessive lethals are silent in the heterozygote. In such a situation, recessive lethality is diagnosed by observing the death of 25 percent of the progeny at some stage of development.
Whether an allele is lethal or not often depends on the environment in which the organism develops. Whereas certain alleles are lethal in virtually any environment, others are viable in one environment but lethal in another. Human hereditary diseases provide some examples. Cystic fibrosis and sickle-
223
Geneticists commonly encounter situations in which expected phenotypic ratios are consistently skewed in one direction because a mutant allele reduces viability. For example, in the cross A/a × a/a, we predict a progeny ratio of 50 percent A/a and 50 percent a/a, but we might consistently observe a ratio such as 55 percent : 45 percent or 60 percent : 40 percent. In such a case, the recessive allele is said to be sublethal because the lethality is expressed in only some but not all of the homozygous individuals. Thus, lethality may range from 0 to 100 percent, depending on the gene itself, the rest of the genome, and the environment.
We have seen that lethal alleles are useful in diagnosing the time at which a gene acts and the nature of the phenotypic defect that kills. However, maintaining stocks bearing lethal alleles for laboratory use is a challenge. In diploids, recessive lethal alleles can be maintained as heterozygotes. In haploids, heat-
Null alleles for genes identified through genomic sequencing can be made by using a variety of “reverse genetic” procedures that specifically knock out the function of that gene. These will be described in Chapter 14.
KEY CONCEPT
To see if a gene is essential, a null allele is tested for lethality.We now turn to the approaches that can be used to detect the interaction between two or more loci.