12.5 Long-Term Inactivation of Genes in a Chromatin Environment
Thus far, we have looked at how genes are activated in a chromatin environment. However, as stated at the beginning of this chapter, most of the genes in eukaryotic genomes are off at any one time. One of the most surprising findings of the genomics era is that many eukaryotic genes are inactive for the life of the organism. This leads to two questions that will be addressed in this section. First, why do organisms have genes that are always inactive? Second, how do organisms keep genes in an inactive state for their entire lifetime?
One of the most useful models for understanding mechanisms that maintain the long-term inactivity of genes concerns the control of mating-type switching in yeast. The components of the yeast mating-type locus were introduced at the end of Section 12.2. Here we continue the story by focusing on the mechanism of mating-type switching, which requires each yeast cell to maintain inactive copies of a and α genes elsewhere in their genome.
Mating-type switching and gene silencing
Haploid yeast cells are able to switch their mating type, sometimes as often as every cell cycle. In this way, a yeast haploid cell of one mating type (say a) will form a colony of both a and α cells that can mate to form diploid (a/α) cells. During times of crisis such as periods of nutrient scarcity, each diploid cell can undergo meiosis and produce four haploid spores. This process is advantageous to the survival of the species because spores can survive adverse environment conditions better than haploid cells.
Genetic analyses of certain mutants that either could not switch or could not mate (they were sterile) were sources of key insights into mating-type switching. Among the switch mutants were several mutant loci, including the HO gene and the HMRa and HMLα genes. Further study revealed that the HO gene encodes an endonuclease, an enzyme that cleaves DNA (see Chapter 10), required for the initiation of switching. It was also found that the HMRa and HMLα loci, which are on the same chromosome as the MAT locus, contain “cassettes” of the MATa and MATα alleles, respectively, that are not expressed. The HMR and HML loci are thus “silent” cassettes. Recall from Chapter 8 that one form of gene silencing occurs when dsRNA targets the RISC complex to destroy complementary RNA. This is an example of post-transcriptional gene silencing. In contrast, HMRa and HMLα cannot be transcribed, and, as such, they are examples of transcriptional gene silencing.
Two features of the mating-type switch were of interest to geneticists: how do cells switch their mating type, and why are HMRa and HMLα transcriptionally silent? The key to switching is the HO endonuclease, which initiates the mating-type switch by generating a double-strand break at the MAT locus. The interconversion of mating type then takes place by a type of recombination between the segment of DNA (a cassette) from one of the two unexpressed loci and the MAT locus. The result is the replacement of the old cassette at the MAT locus with a new cassette from either HMRa or HMLα. The resulting mating type is either the MATa or the MATa type, depending on which gene is at the MAT locus (Figure 12-22). The inserted cassette is actually copied from the HMRa or HMLα locus. In this manner, the switch is reversible because the information for the a and α cassettes is always present at the HMRa and HMLα loci and never lost. Thus, the mating switch provides one example of genes that need to be silenced for the entire lifetime of an organism. As you will see later in this chapter, the silencing of genes for an entire lifetime also occurs in humans and all other mammals.
Figure 12-22: Mating-type switching is controlled by recombination of DNA cassettes
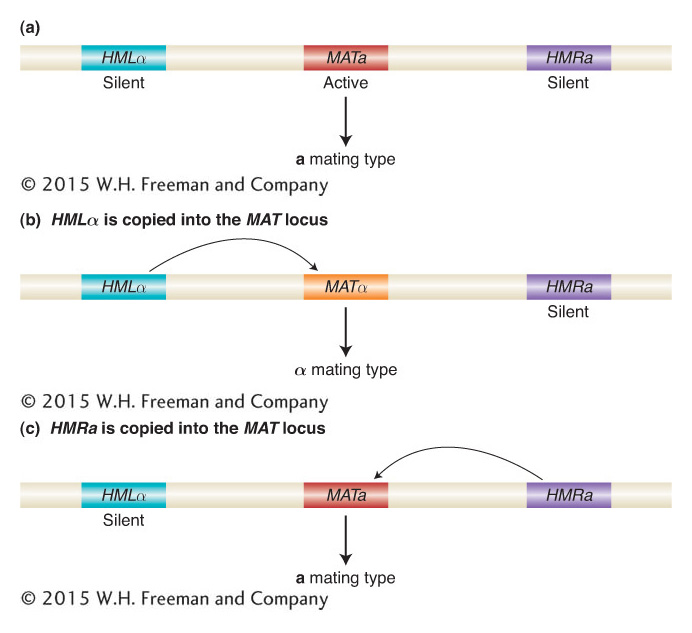
Figure 12-22: S. cerevisiae chromosome III encodes three mating-type loci, but only the genes at the MAT locus are expressed. HML encodes a silent cassette of the α genes, and HMR encodes a silent cassette of the a genes. Copying of a silent cassette and insertion through recombination at the MAT locus switches mating type.
Page 455
The second feature of mating-type switching of interest to geneticists is the mechanism underlying gene silencing. Why are genes in the HMRa and HMLα cassettes not expressed? Normally, these cassettes are “silent.” However, in SIR mutants (silent information regulators), silencing is compromised such that both a and α information is expressed. The resulting mutants are sterile. This means that in normal, nonmutant yeast, genes at the HMRa and HMLα cassettes are capable of being expressed but are not because of the action of the Sir proteins. The Sir2, Sir3, and Sir4 proteins form a complex that plays a key role in gene silencing. Sir2 is a histone deacetylase that facilitates the condensation of chromatin and helps lock up HMRa and HMLα in chromatin domains where transcription cannot be initiated.
Gene silencing is a very different process from gene repression: silencing is a position effect that depends on the neighborhood in which genetic information is located. For example, a normally active gene inserted into the HMR or HML loci would be silenced. You will learn more about position effects later, in the section on position-effect variegation in the fruit fly Drosophila melanogaster.
Heterochromatin and euchromatin compared
Let’s examine why long-term gene silencing, of the type that silences HMLα and HMRa, is a different process from gene repression. To do so, it is important to note that chromatin is not uniform over all chromosomes: certain regions of chromosomes are bundled in highly condensed chromatin called heterochromatin. Other domains are packaged in less-condensed chromatin called euchromatin (see Figure 12-11b). Chromatin condensation changes in the course of the cell cycle. The chromatin of cells entering mitosis becomes highly condensed as the chromosomes align in preparation for cell division. After cell division, regions forming heterochromatin remain condensed, especially around the centromeres and telomeres (called constitutive heterochromatin), whereas the regions forming euchromatin become less condensed. As seen for the β-interferon example (see Figure 12-19), the chromatin of active genes can change in response to developmental stage or environmental conditions.
Page 456
The major distinction between heterochromatin and euchromatin is that the former contains few genes while the latter is rich in genes. But what is heterochromatin if not genes? Most of the eukaryotic genome is composed of repetitive sequences that do not make protein or structural RNA (see Chapter 14). Thus, the densely packed nucleosomes of heterochromatin (organized into 30-nm chromatin fibers; see Figure 12-11a) are said to form a “closed” structure that is largely inaccessible to regulatory proteins and inhospitable to gene activity. In contrast, euchromatin, with its more widely spaced nucleosomes (organized into 10-nm fibers; see Figure 12-11a), assumes an “open” structure that permits transcription.
KEY CONCEPT
The chromatin of eukaryotes is not uniform. Highly condensed heterochromatic regions have fewer genes and lower recombination frequencies than do the less-condensed euchromatic regions.
Position-effect variegation in Drosophila reveals genomic neighborhoods
Long before the silent-mating loci of yeast were described, geneticist Hermann Muller discovered an interesting genetic phenomenon while studying Drosophila: chromosomal neighborhoods exist that can silence genes that are experimentally “relocated” to adjacent regions of the chromosome. In these experiments, flies were irradiated with X rays to induce mutations in their germ cells. The progeny of the irradiated flies were screened for unusual phenotypes. A mutation in the white gene, near the tip of the X chromosome, will result in progeny with white eyes instead of the wild-type red color. Some of the progeny had very unusual eyes with patches of white and red color. Cytological examination revealed a chromosomal rearrangement in the mutant flies: present in the X chromosome was an inversion of a piece of the chromosome carrying the white gene (Figure 12-23). Inversions and other chromosomal rearrangements will be discussed in Chapter 17. In this rearrangement, the white gene, which is normally located in a euchromatic region of the X chromosome, now finds itself near the heterochromatic centromere. In some cells, the heterochromatin can “spread” to the neighboring euchromatin and silence the white gene. Patches of white tissue in the eye are derived from the descendants of a single cell in which the white gene has been silenced and remains silenced through future cell divisions. In contrast, the red patches arise from cells in which heterochromatin has not spread to the white gene, and so this gene remains active in all its descendants. The existence of red and white patches of cells in the eye of a single organism dramatically illustrates two features of epigenetic silencing. First, that the expression of a gene can be repressed by virtue of its position in the chromosome rather than by a mutation in its DNA sequence. Second, that epigenetic silencing can be inherited from one cell generation to the next.
Figure 12-23: Spreading heterochromatin can silence genes
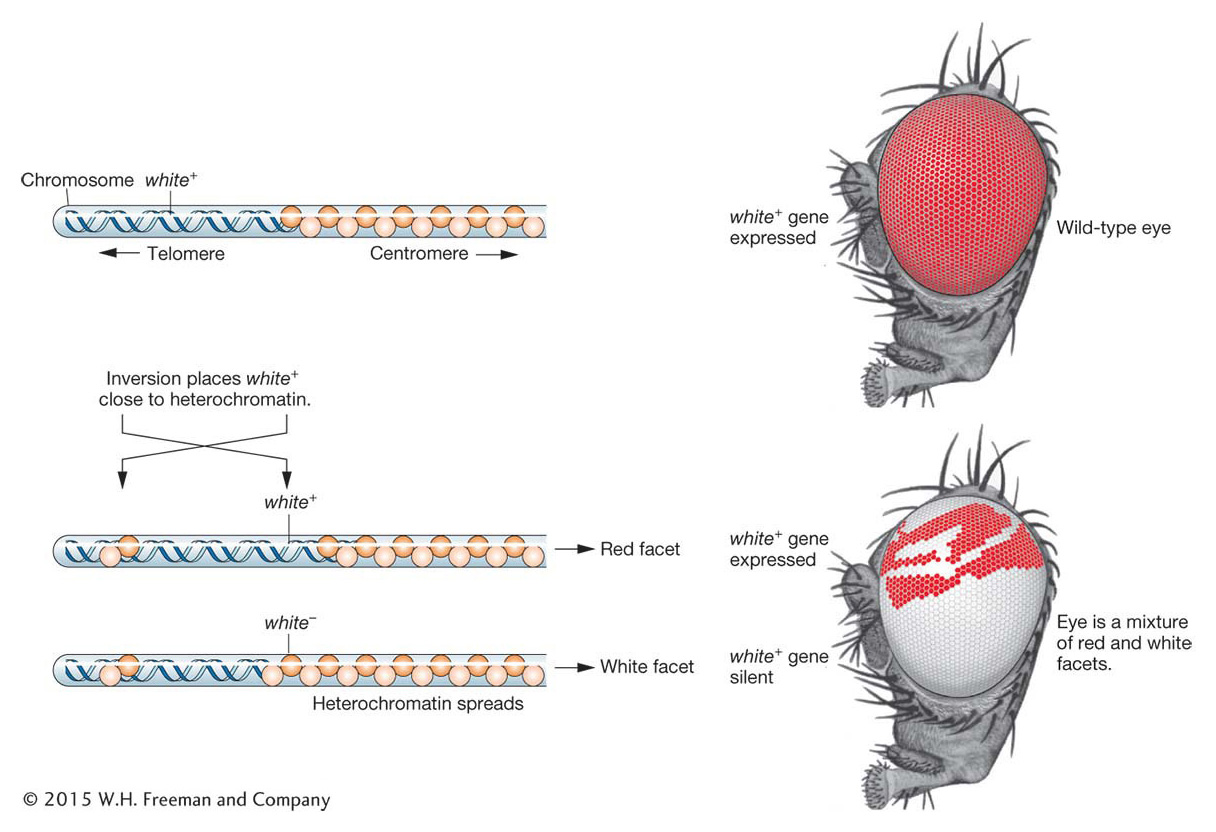
Figure 12-23: Chromosomal rearrangement produces position-effect variegation. Chromosomal inversion places the wild-type white allele close to heterochromatin. The spread of heterochromatin silences the allele. Eye facets are white instead of the wild-type red wherever the allele has been silenced.
Findings from subsequent studies in Drosophila and yeast demonstrated that many active genes are silenced in this mosaic fashion when they are relocated to neighborhoods (near centromeres or telomeres) that are heterochromatic. Thus, the ability of heterochromatin to spread into euchromatin and silence genes is a feature common to many organisms. This phenomenon has been called position-effect variegation (PEV). It provides powerful evidence that chromatin structure is able to regulate the expression of genes—in this case, determining whether genes with identical DNA sequence will be active or silenced.
Page 457
KEY CONCEPT
Active genes that are relocated to genomic neighborhoods that are heterochromatic may be silenced if the heterochromatin spreads to the genes.
Genetic analysis of PEV reveals proteins necessary for heterochromatin formation
WHAT GENETICISTS ARE DOING TODAY
Geneticists reasoned that PEV could be exploited to identify the proteins necessary for forming heterochromatin. To this end, they isolated mutations at a second chromosomal locus that either suppressed or enhanced the variegated pattern (Figure 12-24). Suppressors of variegation [called Su(var) ] are genes that, when mutated, reduce the spread of heterochromatin, meaning that the wild-type products of these genes are required for spreading. In fact, the Su(var) alleles have proved to be a treasure trove for scientists interested in the proteins that are required to establish and maintain the inactive, heterochromatic state. Among more than 50 Drosophila gene products identified by these screens was heterochromatin protein-1 (HP-1), which had previously been found associated with the heterochromatic telomeres and centromeres. Thus, it makes sense that a mutation in the gene encoding HP-1 will show up as a Su(var) allele because the protein is required in some way to generate the higher-order chromatin structure associated with heterochromatin.
Figure 12-24: Some gene products enhance or suppress the spread of heterochromatin
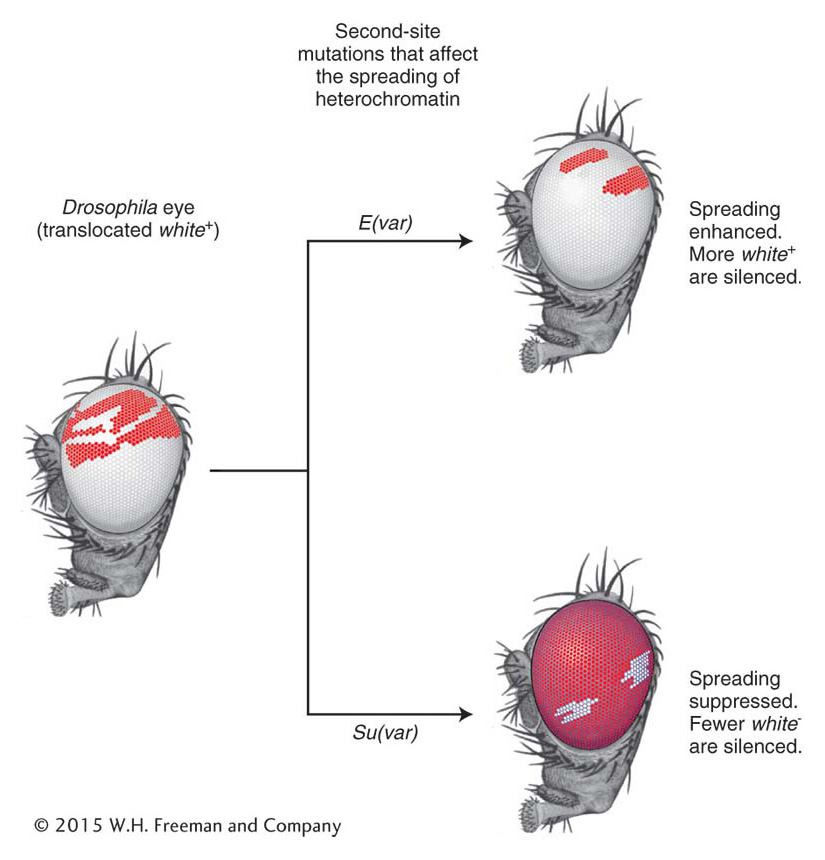
Figure 12-24: Mutations were used to identify genes that suppress, Su(var), or enhance, E(var), position-effect variegation.
Page 458
But why does HP-1 bind to some DNA regions and not others? The answer to that came with the discovery that another Su(var) gene encoded a methyltransferase that adds methyl groups to lysine 9 in the tail of histone H3 (called histone H3 methyltransferase or HMTase). Recall that H3K9me is associated with the repression of gene expression (see Figure 12-13b). This is because chromatin modified in this way binds HP-1 proteins, which then associate to form heterochromatin. Proteins similar to HP-1 and HMTase have been isolated in diverse taxa, suggesting the conservation of an important eukaryotic function.
We have seen that actively transcribed regions are associated with nucleosomes whose histone tails are hyperacetylated and that transcriptional activators such as GCN5 encode a histone acetyltransferase activity. As already discussed, acetyl marks can also be removed from histones by histone deacetylases. Similarly, chromatin made up of nucleosomes that are methylated at H3K9 and bound up with HP-1 protein contain epigenetic marks that are associated with heterochromatin. Scientists are now able to separate heterochromatin and euchromatin and analyze differences in histone modifications and bound proteins. The procedure used, chromatin immunoprecipitation (ChIP), is described in Chapter 14.
Figure 12-25 illustrates that, in the absence of any barriers, heterochromatin might spread into adjoining regions and inactivate genes in some cells but not in others. It could be what is happening to the white gene of Drosophila when it is translocated near the domain of heterochromatin associated with the chromosome ends. But can the spread of heterochromatin be stopped? One can imagine that the spreading of heterochromatin into active gene regions could be disastrous for an organism because active genes would be silenced as they are converted into heterochromatin. To avert this potential disaster, the genome contains DNA elements called barrier insulators that prevent the spreading of heterochromatin by creating a local environment that is not favorable to heterochromatin formation. For example, a barrier insulator could bind HATs and, in doing so, make sure that the adjacent histones are hyperacetylated. A model for how a barrier insulator might act to “protect” a region of euchromatin from being converted into heterochromatin is shown in Figure 12-26.
Figure 12-25: Heterochromatin may spread farther in some cells than in others

Figure 12-25: The spread of heterochromatin into adjacent euchromatin is variable. In four genetically identical diploid cells, heterochromatin spreads enough to knock out a gene in some chromosomes but not others. Heterochromatin and euchromatin are represented by orange and green spheres, respectively.
Figure 12-26: Barrier insulators stop the spread of heterochromatin
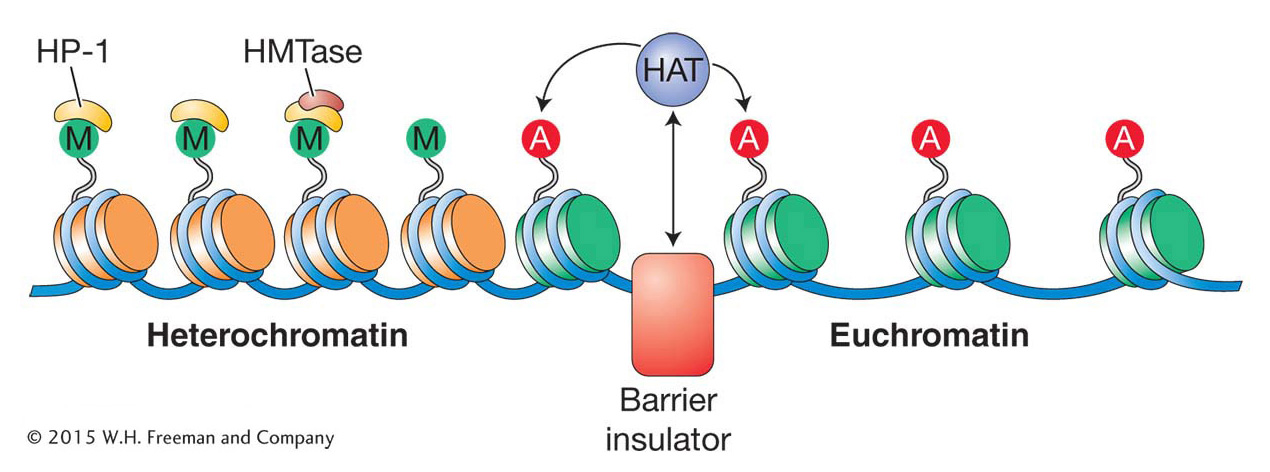
Figure 12-26: In this model, barrier insulators recruit enzymatic activities such as histone acetyltransferase (HAT) that promote euchromatin formation. The letter “M” stands for methylation, and the letter “A” for acetylation.
Page 459
KEY CONCEPT
The isolation of critical proteins necessary for the formation of heterochromatin, including HP-1 and HMTase, was made possible by the isolation of mutant strains of Drosophila that suppressed or enhanced PEVPage 460




