Chapter 4. From GloFish to human disease models: Forward and reverse genetics in zebrafish
Introduction
From GloFish to human disease models: Forward and reverse genetics in zebrafish
Introduction
The zebrafish (Danio rerio) is not only a popular tropical fish pet but also an important model organism for vertebrate development, genetics, and disease. Zebrafish have certain features—
The Model Organism Zebrafish

Oppenheimer photo courtesy of Dr. Marnie Halpern. Streisinger photo from Nature Reviews Genetics 3, 717–
First described by Hamilton in 18221, the zebrafish is native to the flood plains of India and lives in diverse water habitats2,3. Zebrafish were imported to Europe in the early 1900s as an aquarium fish4. Within twenty to thirty years, zebrafish became highly popular pets among hobbyists because they are easy to care for and because they have beautiful, diverse natural pigment patterns. Along with the features that made zebrafish popular pets, these animals also have many attributes of excellent model systems for scientific inquiry. Zebrafish are small (2-

Figure 1 Source: Figure 3 (Panel A only) from Garcia-
Since the 1930s, zebrafish, and other teleost species, have been used as model systems for understanding the developmental basis of vertebrate embryology. Notably, Dr. Jane Marion Oppenheimer (Figure 1a) published a series of seven critically important papers using fish. In one study, she demonstrated that a developmental structure called the dorsal organizer (or Spemann organizer) is responsible for initiating the body plan in vertebrates5. This developmental structure is functionally conserved between fish and amphibians5.

Images courtesy of Dr. Keith Cheng. File name: 3-
Dr. George Streisinger (Figure 1b) and colleagues at the University of Oregon launched the modern era of zebrafish genetics. They recognized the strengths of zebrafish in developmental biology and established zebrafish as a model system for vertebrate genetics6. In the 1970s, Streisinger purchased a “golden” zebrafish, one of the first mutant zebrafish strains, from a local pet shop. This “golden” zebrafish produces pigment similar to normal striped zebrafish, but the pigment intensity is much lighter (Figure 2). The recessive nature of the “golden” allele provided a simple, visible phenotype that served as a test allele for the development of new genetic tools7.
1.Hamilton (formerly Buchanan), F. An account of the fishes found in the River Ganges and its branches. Edinburgh, UK: Constable and Co., 1822.
2.Engeszer, R. E. et al. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish 4, 21–40 (2007). doi: 10.1089/zeb.2006.9997
3.Spence, R. et al. The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews of the Cambridge Philosophical Society 83, 13–34 (2008). doi: 10.1111/j.1469-
4.Stansch, K. Die exotischen zierfische in wort und bild. Braunschweig, Germany: Gustav, Wenzel & Sohn, 1914, 349.
5.Oppenheimer, J. M. Structures developed in amphibians by implantation of living fish organizer. Proceedings of the Society for Experimental Biology and Medicine 34, 461–463 (1936). doi: 10.3181/00379727-
6.Grunwald, D. J. & Eisen, J. S. Headwaters of the zebrafish—emergence of a new model vertebrate. Nature Reviews Genetics 3, 717–724 (2002). doi: 10.1038/nrg892
7.Streisinger, G. et al. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293–296 (1981). doi: 10.1038/291293a0
Naturally Occurring Mutations in Zebrafish
Zebrafish display a number of naturally occurring mutations. Many fish carrying these mutations were isolated from the wild and are now sold in pet stores. These naturally occurring mutations include pigmentation mutations such as leopard and brass and developmental mutations such as the long fin allele.
Forward Genetics Approaches in Zebrafish
How can a scientist identify a gene responsible for a phenotype observed in zebrafish? As discussed in Chapter 2 of your textbook, forward genetics is an approach that starts with a phenotype of interest and identifies the gene or genes responsible for the phenotype. Over the years, researchers have developed a wide range of techniques to produce new mutations in zebrafish. The first method involved gamma ray irradiation of zebrafish sperm or early zebrafish embryos. Gamma rays are an effective mutagen (an agent that produces mutations), and they disrupt genes in numerous ways. Gamma ray irradiation produces both small (single-
Because gamma ray irradiation-
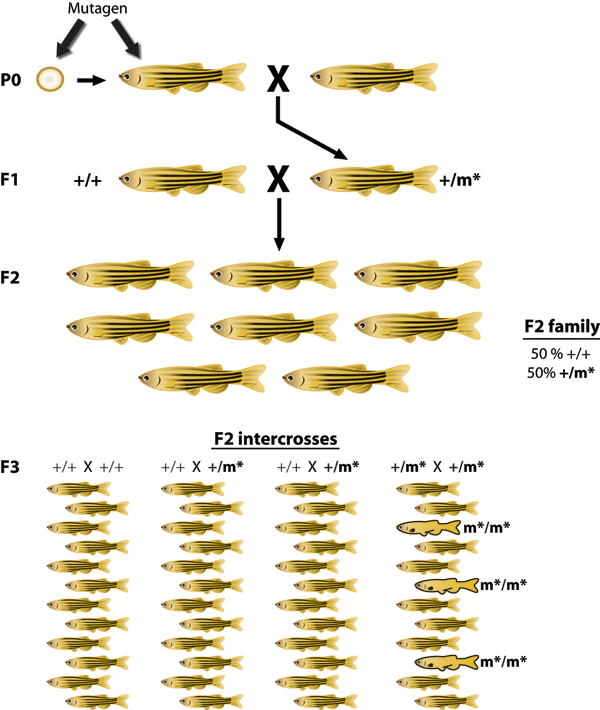
Generated by Illustrator. File name: 3-
To understand the genetic basis of a specific phenotype, researchers typically carry out genetic mapping and positional cloning experiments. These approaches allow researchers to pinpoint the location of the associated gene and identify the responsible mutation. These experiments are much easier to conduct if the genome sequence of the model organism is known. Once scientists recognized the genetic potential of the zebrafish, they began sequencing the zebrafish genome in 2000 at the Sanger Center, UK. The final draft sequence of the zebrafish genome was released in 2011. As the zebrafish genome was sequenced, hundreds of zebrafish mutations were isolated. For example, in 2005, scientists learned that the “golden” phenotype was caused by a mutation in the SLC24A5 gene10. Could this gene tell us anything about humans? Interestingly, a mutation in the human SLC24A5 gene is associated with light skin color and is most often found in people of northern and western European descent. This observation highlights the strong degree of conservation of gene function between zebrafish and humans and provides support for using zebrafish as a model system for medical science.
Genetic engineering of the zebrafish initially started with a low efficiency but simple method of injecting DNA solutions into fertilized zebrafish eggs. In rare cases, this process results in integration of the injected DNA into zebrafish chromosomes. This method was used to generate the first transgenic pets—
Scientists developed two additional technologies—
The development of mobile DNA elements called transposons (discussed in Chapter 15 of your textbook) made microinjection and integration of DNA into the zebrafish genome highly efficient and accessible to a broad range of researchers14. With DNA transposon-
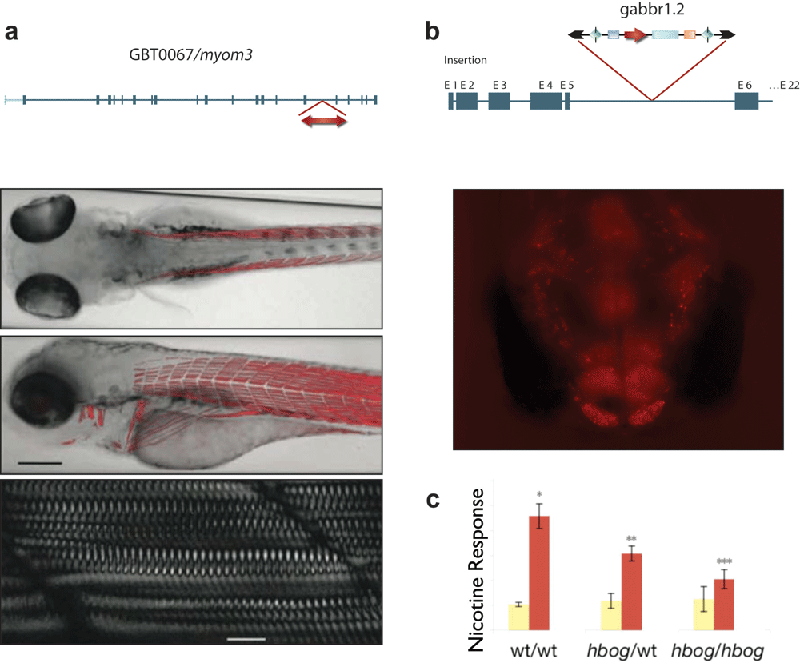
(a) From Figure 3C of Clark, K. J. et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nature Methods 8, 506–
8.Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996).
9.Driever, W. et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 (1996).
10.Lamason, R. et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310, 1782–1786 (2005). doi: 10.1126/science.1116238
11.glofish.com. <http://www.glofish.com>
12.Gaiano, N. et al. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 383, 829–832 (1996). doi: 10.1038/383829a0
13.Amsterdam, A. et al. Identification of 315 genes essential for early zebrafish development. Proceedings of the National Academy of Sciences of the United States of America 101, 12792–12797 (2004). doi: 10.1073/pnas.0403929101
14.Ni, J. et al. Transposon tools hopping in vertebrates. Briefings in Functional Genomics and Proteomics 7, 444–453 (2008). doi: 10.1093/bfgp/eln049
15.Clark, K. J. et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nature Methods 8, 506–512 (2011). doi: 10.1038/nmeth.1606
16.Petzold, A. M. et al. Nicotine response genetics in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America 106, 18662–18667 (2009). doi: 10.1073/pnas.0908247106
Reverse Genetics Approaches in Zebrafish
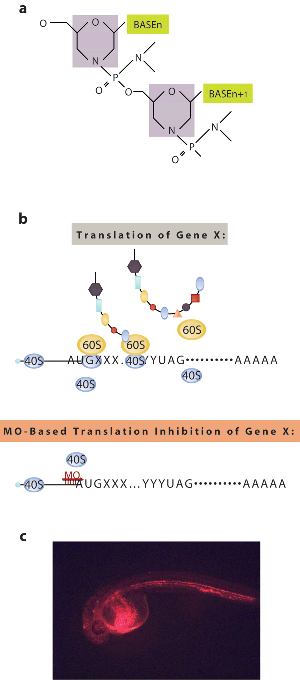
Figure 5 Source: (a) and (b) are from Figure 1a and Figure 2a of Ekker, S. C. & Larson, J. D. Morphant technology in model developmental systems. Genesis 30, 89–
As discussed in Chapter 2 and Section 14.7 of your textbook, reverse genetics is an approach that starts with a gene of unknown function and then identifies its function or associated phenotypes. The most widely used reverse genetic tool in zebrafish is morpholino antisense technology, which is used to knock down gene expression (Figure 5a)17. Morpholinos are synthetic nucleotide sequences that have a unique backbone chemistry making them undetectable and unmodifiable by cells. Unlike siRNA-
17.Bill, B. R. et al. A primer for morpholino use in zebrafish. Zebrafish 6, 69–77 (2009). doi: 10.1089/zeb.2008.0555
18.Nasevicius, A. & Ekker, S. C. Effective targeted gene “knockdown” in zebrafish. Nature Genetics 26, 216–220 (2000). doi: 10.1038/79951
Generating Zebrafish Mutants Using High-
One goal of the zebrafish research community is using a combination of forward and reverse genetic approaches to produce a mutation in every gene of the organism. ENU mutants are currently being screened using high-
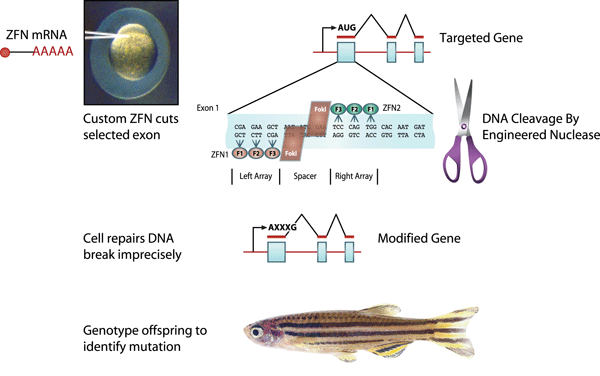
Figure 6 Source: Adapted from Ekker, S. C. Zinc finger-
Until recently, the most commonly used mutagenesis approaches were largely random or semirandom in nature. Today, however, scientists are using new approaches that target specific gene sequences to create double-
19.Moens, C. B. et al. Reverse genetics in zebrafish by TILLING. Briefings in Functional Genomics and Proteomics 7, 454–459 (2008). doi: 10.1093/bfgp/eln046
15.Clark, K. J. et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nature Methods 8, 506–512 (2011). doi: 10.1038/nmeth.1606
20.Ekker, S. C. Zinc finger-
Zebrafish as a Model System: An Ever-
The high conservation of gene function between zebrafish and humans has led to the exponential use of zebrafish in the scientific literature, beginning with the publication of the first large-
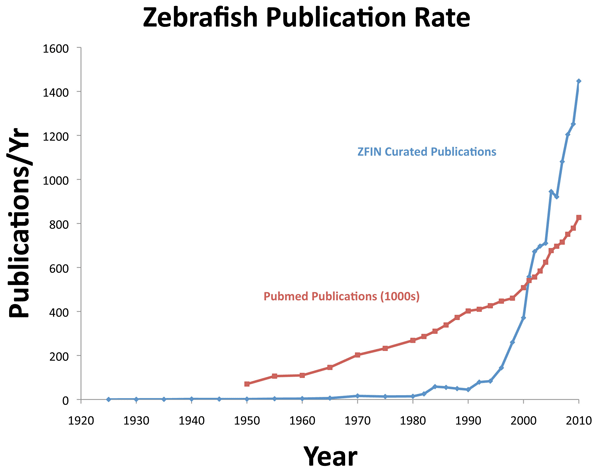
Figure 7 Source: Curated zebrafish publication data were retrieved from http://zfin.org in July 2011, and Pubmed publication data were retrieved from http://www.ncbi.nlm.nih.gov/pubmed in July 2011 and analyzed by the authors.
8.Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996).
9.Driever, W. et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 (1996).
Summary
The zebrafish has a diverse history from aquarium pets to embryology. Recent advances in molecular genetics include the popular use of the zebrafish as a model system for understanding human health. Forward genetic screening using both chemical and insertional mutagens has identified an array of new genetic loci for vertebrate development, disease, and behavior. Reverse genetic approaches, including both targeted gene knockdown and knockouts, now open the door for hypothesis-
References
1.Hamilton (formerly Buchanan), F. An account of the fishes found in the River Ganges and its branches. Edinburgh, UK: Constable and Co., 1822.
2.Engeszer, R. E. et al. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish 4, 21–40 (2007). doi: 10.1089/zeb.2006.9997
3.Spence, R. et al. The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews of the Cambridge Philosophical Society 83, 13–34 (2008). doi: 10.1111/j.1469-
4.Stansch, K. Die exotischen zierfische in wort und bild. Braunschweig, Germany: Gustav, Wenzel & Sohn, 1914, 349.
5.Oppenheimer, J. M. Structures developed in amphibians by implantation of living fish organizer. Proceedings of the Society for Experimental Biology and Medicine 34, 461–463 (1936). doi: 10.3181/00379727-
6.Grunwald, D. J. & Eisen, J. S. Headwaters of the zebrafish—emergence of a new model vertebrate. Nature Reviews Genetics 3, 717–724 (2002). doi: 10.1038/nrg892
7.Streisinger, G. et al. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293–296 (1981). doi: 10.1038/291293a0
8.Haffter, P. et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 (1996).
9.Driever, W. et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 (1996).
10.Lamason, R. et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310, 1782–1786 (2005). doi: 10.1126/science.1116238
11.glofish.com. <http://www.glofish.com>
12.Gaiano, N. et al. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 383, 829–832 (1996). doi: 10.1038/383829a0
13.Amsterdam, A. et al. Identification of 315 genes essential for early zebrafish development. Proceedings of the National Academy of Sciences of the United States of America 101, 12792–12797 (2004). doi: 10.1073/pnas.0403929101
14.Ni, J. et al. Transposon tools hopping in vertebrates. Briefings in Functional Genomics and Proteomics 7, 444–453 (2008). doi: 10.1093/bfgp/eln049
15.Clark, K. J. et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nature Methods 8, 506–512 (2011). doi: 10.1038/nmeth.1606
16.Petzold, A. M. et al. Nicotine response genetics in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America 106, 18662–18667 (2009). doi: 10.1073/pnas.0908247106
17.Bill, B. R. et al. A primer for morpholino use in zebrafish. Zebrafish 6, 69–77 (2009). doi: 10.1089/zeb.2008.0555
18.Nasevicius, A. & Ekker, S. C. Effective targeted gene “knockdown” in zebrafish. Nature Genetics 26, 216–220 (2000). doi: 10.1038/79951
19.Moens, C. B. et al. Reverse genetics in zebrafish by TILLING. Briefings in Functional Genomics and Proteomics 7, 454–459 (2008). doi: 10.1093/bfgp/eln046
20.Ekker, S. C. Zinc finger-