Table : table 2.1 Chemical Bonds and Interactions
| Name | Basis of interaction | Structure | Bond energya |
|---|---|---|---|
| Covalent bond | Sharing of electron pairs | 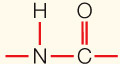 | 5 |
| Ionic attraction | Attraction of opposite charges | 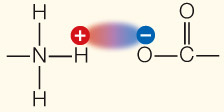 | |
| Hydrogen bond | Electrical attraction between a covalently bonded H atom and an electronegative atom | 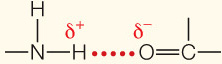 | |
| Hydrophobic interaction | Interaction of nonpolar substances in the presence of polar substances (especially water) | 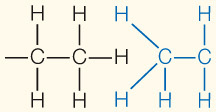 | |
| van der Waals interaction | Interaction of electrons of nonpolar substances | 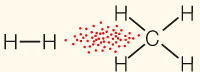 | 1 |
aBond energy is the amount of energy in kcal/mol needed to separate two bonded or interacting atoms under physiological conditions. | |||