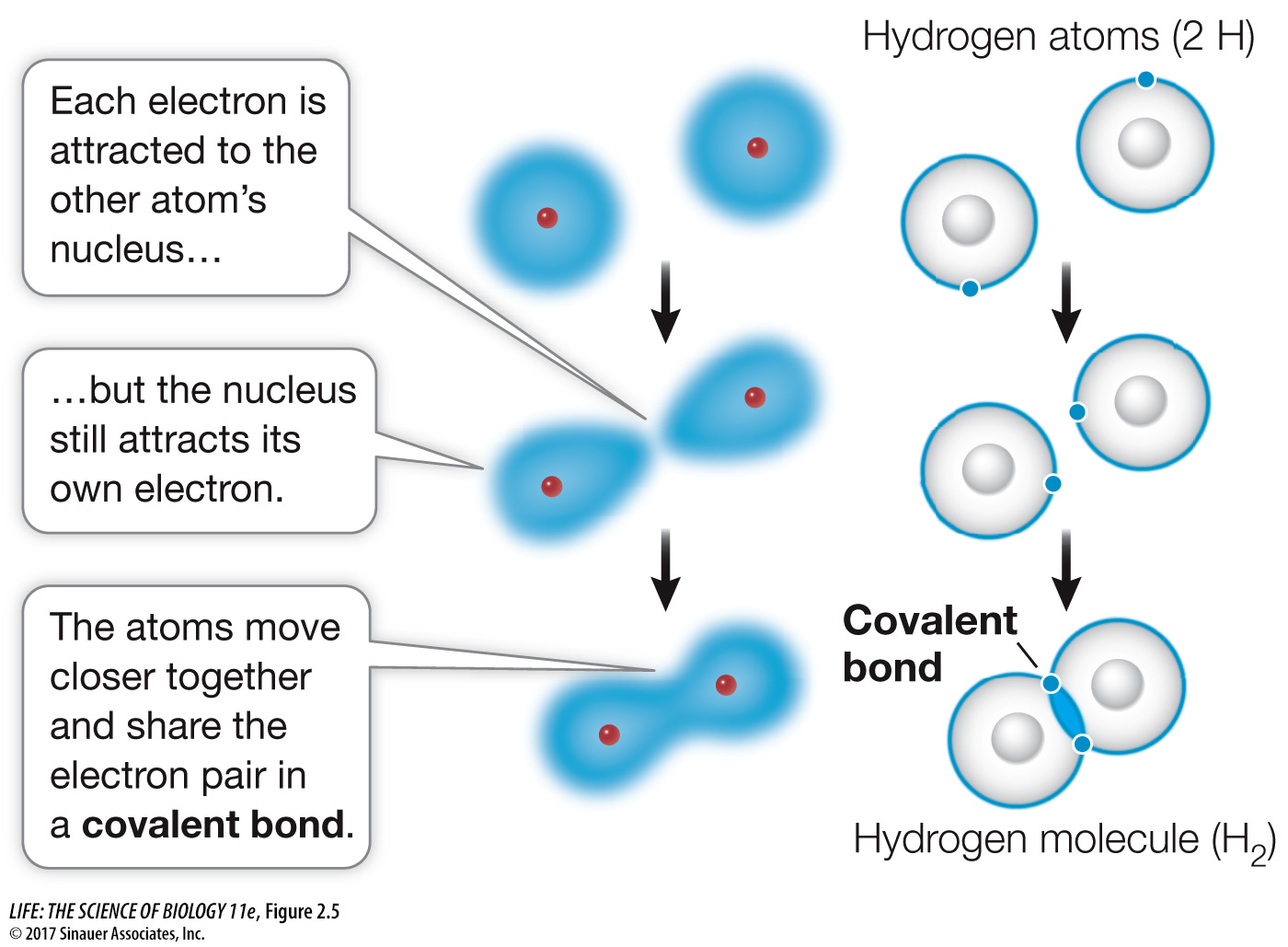
Covalent bonds consist of shared pairs of electrons
A covalent bond forms when two atoms attain stable electron numbers in their outermost shells by sharing one or more pairs of electrons. Consider two hydrogen atoms coming close to one another, each with an unpaired electron in its single shell (Figure 2.5). When the electrons pair up, a stable association is formed, and this links the two hydrogen atoms in a covalent bond, forming the molecule H2.
A compound is a pure substance made up of two or more different elements bonded together in a fixed ratio. Chemical symbols identify the different elements in a compound, and subscript numbers indicate how many atoms of each element are present (e.g., H2O has two atoms of hydrogen bonded to a single oxygen atom). Every compound has a molecular weight that is the sum of the atomic weights of all atoms in the molecule. Looking at the periodic table in Figure 2.2, you can calculate the molecular weight of water to be 18.01. (But remember that this value comes from the average atomic weights of hydrogen and oxygen; the molecular weight of the heavy water in our opening story is higher because it is formed from heavier isotopes.) Molecules that make up living organisms can have molecular weights of up to half a billion.
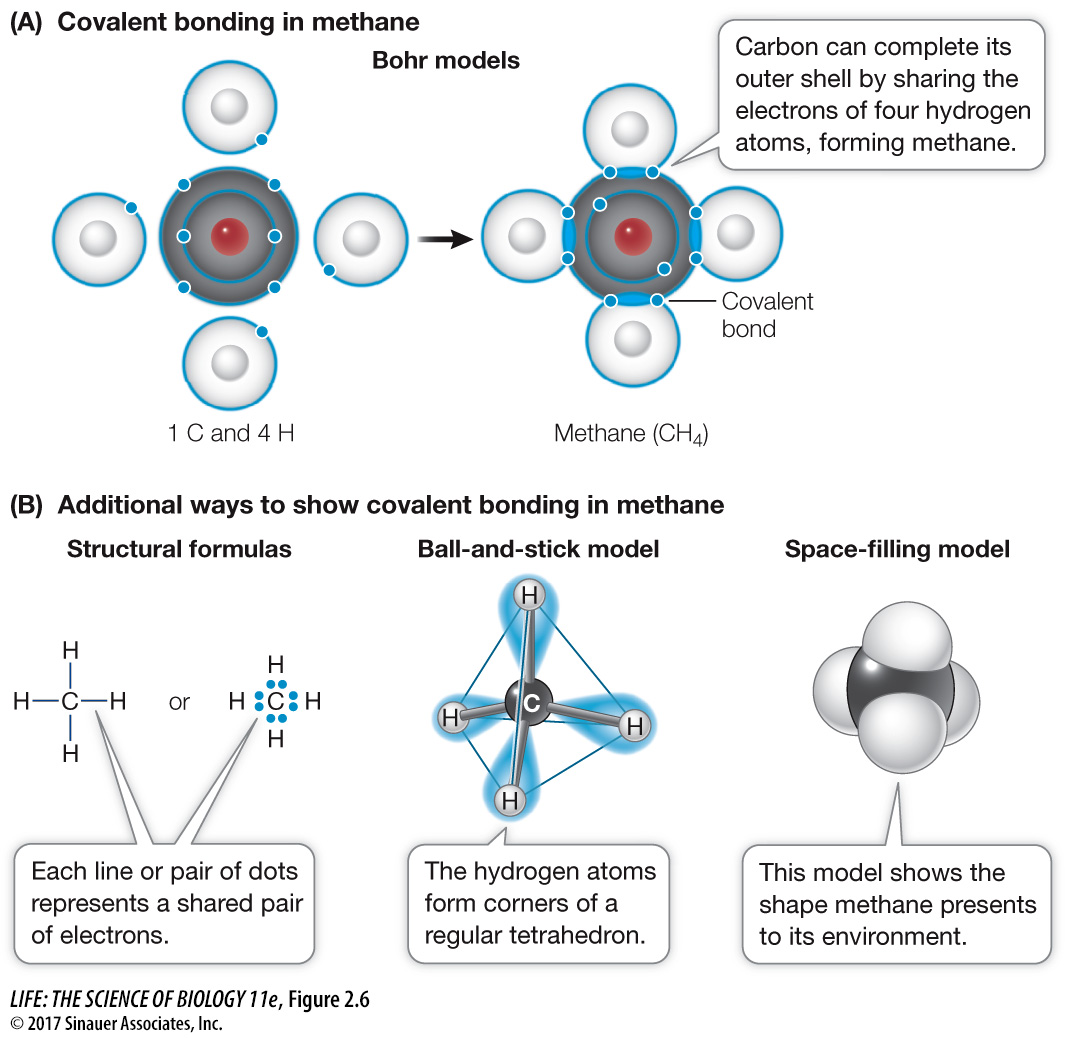
How are the covalent bonds formed in a molecule of methane gas (CH4)? The carbon atom has six electrons: two electrons fill its inner shell, and four unpaired electrons travel in its outer shell. Because its outer shell can hold up to eight electrons, carbon can share electrons with up to four other atoms—
focus: key figure
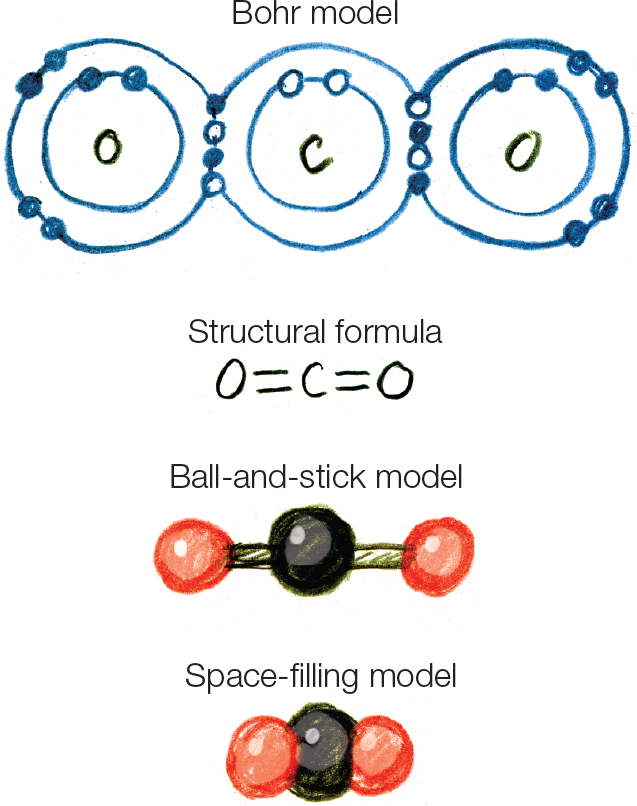
Q: Draw the four representations for carbon dioxide.
| Element | Usual number of covalent bonds |
|---|---|
| Hydrogen (H) | 1 |
| Oxygen (O) | 2 |
| Sulfur (S) | 2 |
| Nitrogen (N) | 3 |
| Carbon (C) | 4 |
| Phosphorus (P) | 5 |
STRENGTH AND STABILITY Covalent bonds are very strong, meaning that it takes a lot of energy to break them. At temperatures where life exists, the covalent bonds of biological molecules are quite stable, as are their three-
ORIENTATION For a given pair of elements—
Even though the orientations of bonds around each atom are fairly stable, the shapes of molecules can change. Think of a single covalent bond as an axle around which the two atoms, along with their other bonded atoms, can rotate.
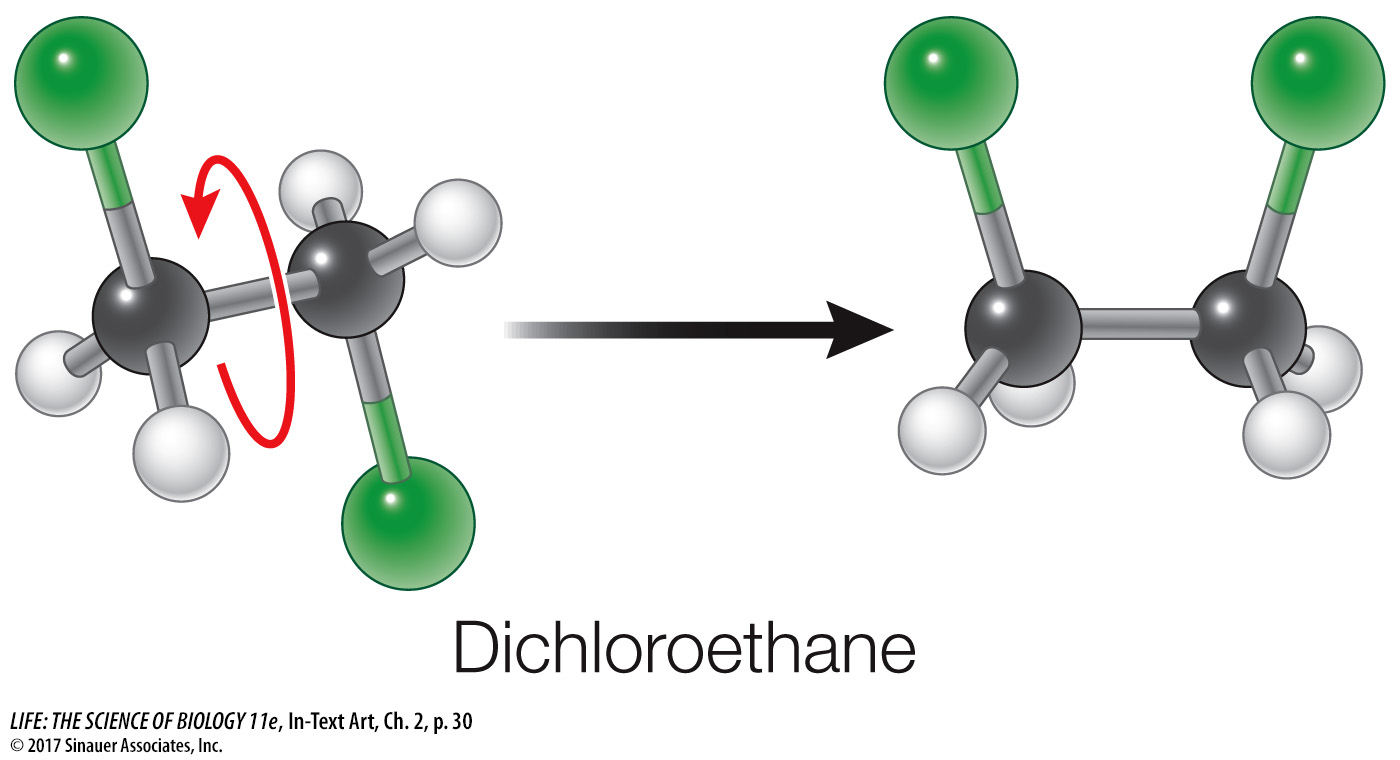
Bond rotation has enormous implications for the large molecules that make up living tissues. SInce long chains of atoms (especially carbons) can rotate freely, there are many possibilities for the arrangement of atoms within the chain. This allows molecules to alter their structures, for example, to fit other molecules.
MULTIPLE COVALENT BONDS Two atoms can share more than one pair of electrons, forming multiple covalent bonds. These can be represented by lines between the chemical symbols for the linked atoms:
A single bond involves the sharing of a single pair of electrons (for example, H─H or C─H).
A double bond involves the sharing of four electrons (two pairs) (C=C).
Triple bonds—
six shared electrons— are rare, but there is one in nitrogen gas (N≡N), which is the major component of the air we breathe.
UNEQUAL SHARING OF ELECTRONS If two atoms of the same element are covalently bonded, there is an equal sharing of the pair(s) of electrons in their outermost shells. However, when the two atoms are of different elements, the sharing is not necessarily equal. One nucleus may exert a greater attractive force on the electron pair than the other nucleus, so that the pair tends to be closer to that atom.
The attractive force that an atomic nucleus exerts on electrons is called its electronegativity. The electronegativity of an atom depends roughly on how many positive charges it has (atoms with more protons are more positive and thus more attractive to electrons) and on the distance between the nucleus and the electrons in the outer (valence) shell (the closer the electrons, the greater the electronegative pull). Table 2.3 shows the electronegativities (which are calculated to produce dimensionless quantities) of some elements important in biological systems. Note that oxygen (O) is very electronegative; in fact, oxygen is the second most electronegative element (after fluorine). Many organisms exploit the negativity of oxygen; moving electrons between C and O atoms powers living systems, as you will see in many subsequent examples in this text.
| Element | Electronegativity |
|---|---|
| Oxygen (O) | 3.5 |
| Chlorine (Cl) | 3.1 |
| Nitrogen (N) | 3.0 |
| Carbon (C) | 2.5 |
| Phosphorus (P) | 2.1 |
| Hydrogen (H) | 2.1 |
| Sodium (Na) | 0.9 |
| Potassium (K) | 0.8 |
If two atoms are close to one another in electronegativity, they will share electrons equally in what is called a nonpolar covalent bond. Two oxygen atoms, for example, each with an electronegativity of 3.5, will share electrons equally. So will two hydrogen atoms (each with an electronegativity of 2.1). But when hydrogen bonds with oxygen to form water, the electrons involved are unequally shared; they tend to be nearer to the oxygen nucleus because it is more electronegative than hydrogen. When electrons are drawn to one nucleus more than to the other, the result is a polar covalent bond (Figure 2.7).
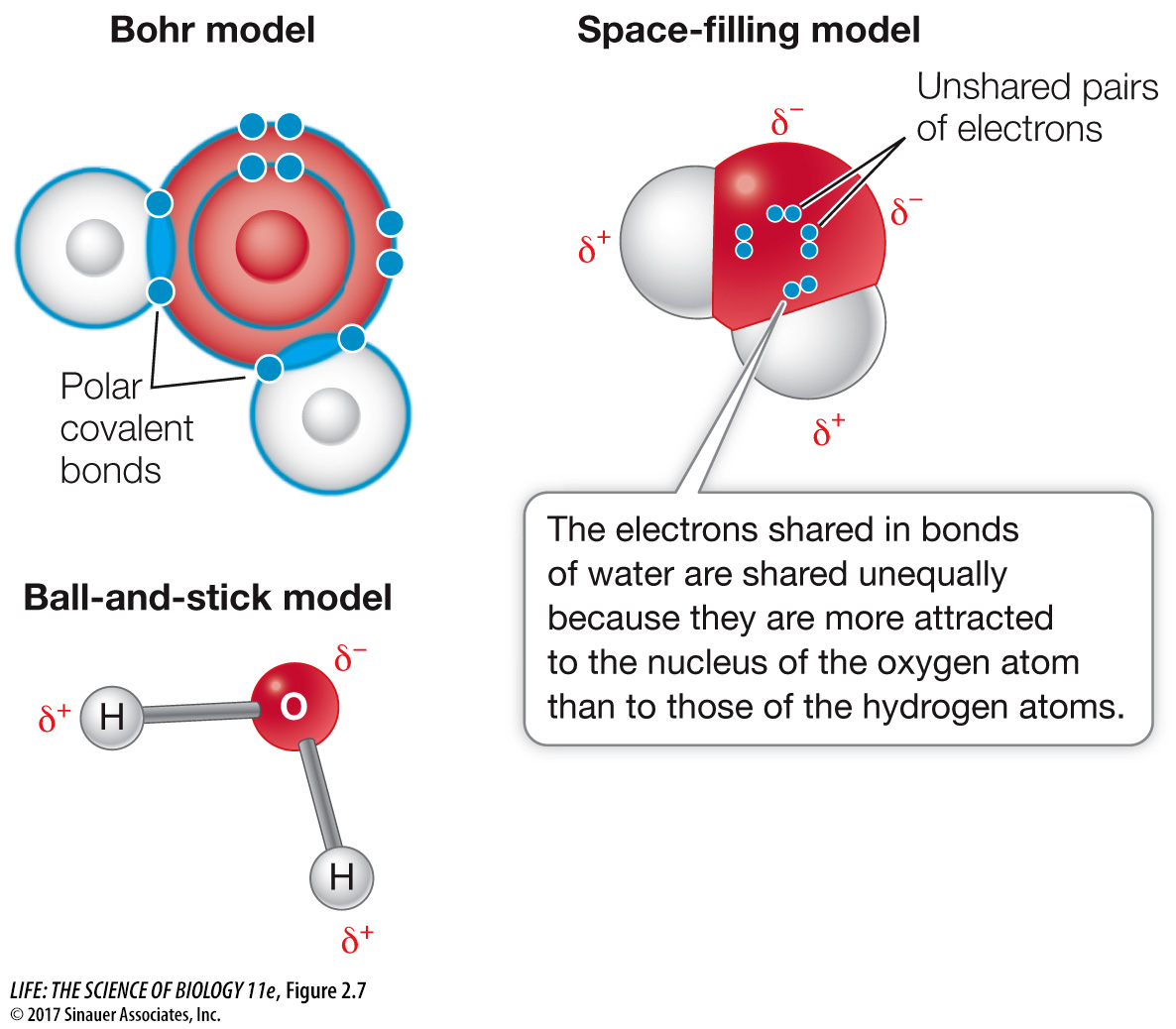
Because of this unequal sharing of electrons, the oxygen end of the hydrogen–