What are atoms?
Each atom consists of a dense, positively charged nucleus, around which one or more negatively charged electrons move (Figure 2.1). The nucleus contains one or more positively charged protons and may contain one or more neutrons with no electric charge.
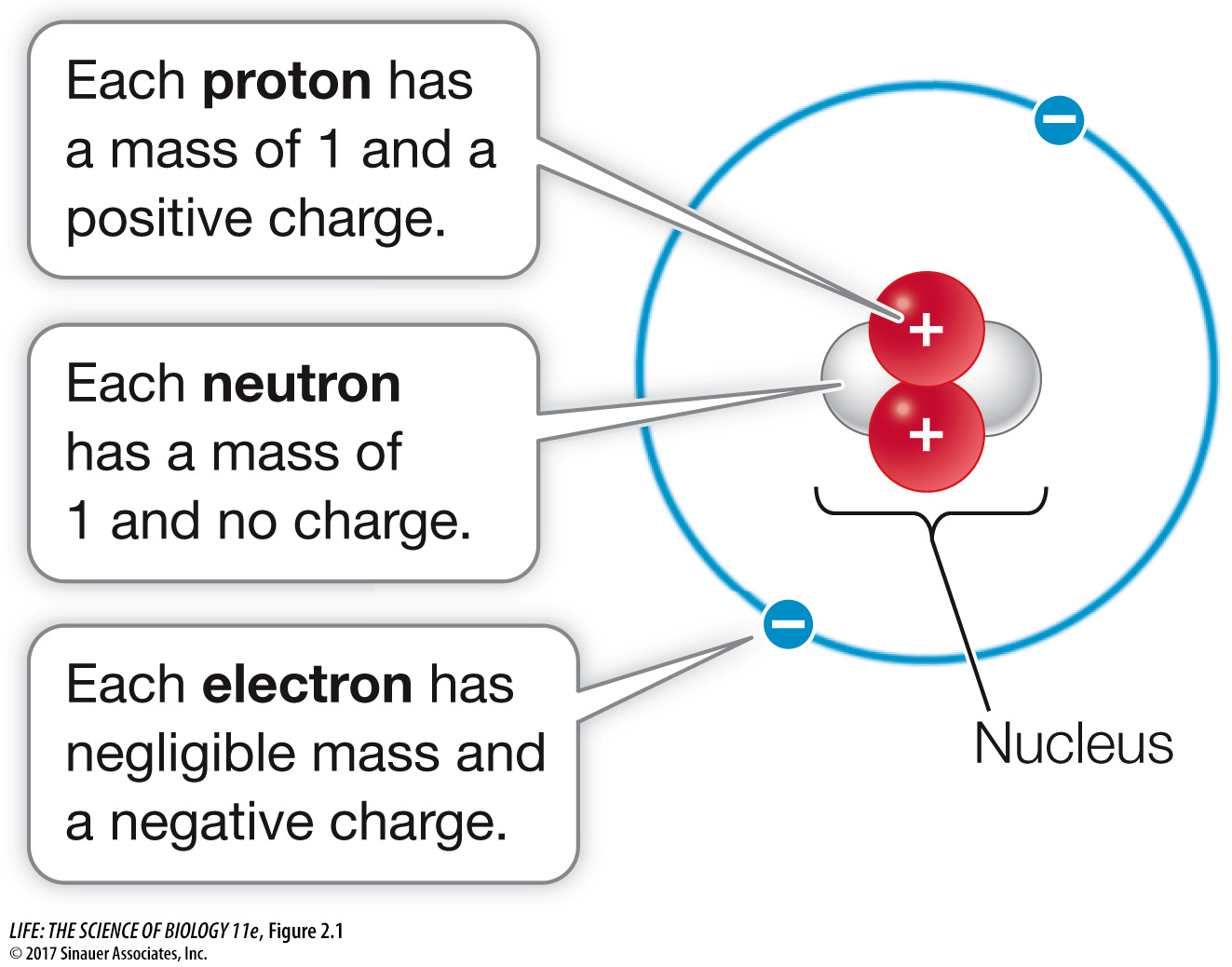
The mass of a proton serves as a standard unit of measure called the dalton (named after the English chemist John Dalton). A single proton or neutron has a mass of about 1 dalton (Da), which is 1.7 × 10–24 grams (0.0000000000000000000000017 g), but an electron is even tinier at 9 × 10–28 g (0.0005 Da). Because the mass of an electron is negligible compared with the mass of a proton or a neutron, the contribution of electrons to the mass of an atom can usually be ignored when measurements and calculations are made. It is electrons, however, that determine how atoms will combine with other atoms to form stable associations.
Each proton has a positive electric charge, defined as +1 unit of charge. An electron has a negative charge equal and opposite to that of a proton (–1). The neutron, as its name suggests, is electrically neutral, so its charge is 0. Charges that are different (+/–) attract each other, whereas charges that are alike (+/+, –/–) repel each other. Generally, atoms are electrically neutral because the number of electrons in an atom equals the number of protons.