Protein shapes can change
As we saw in the case of hemoglobin, which undergoes subtle shape changes when it binds oxygen, the shapes of proteins can change as a result of their interactions with other molecules. Proteins can also change shape if they undergo covalent modifications.
Proteins interact with other molecules. Proteins do not exist in isolation. In fact, if a biochemist “goes fishing” with a particular protein, by attaching the protein to a chemical “hook” and inserting it into cells, the protein will often be attached to something else when it is “reeled in.” These molecular interactions are reminiscent of the interactions that make up quaternary structure (see above). If a polypeptide comes into contact with another molecule, R groups on its surface may form weak interactions (e.g., hydrophobic, van der Waals) with groups on the surface of the other molecule. This may disrupt some of the interactions between R groups within the polypeptide, causing it to undergo a change in shape (Figure 3.13A). You will see many instances of this in the coming chapters.
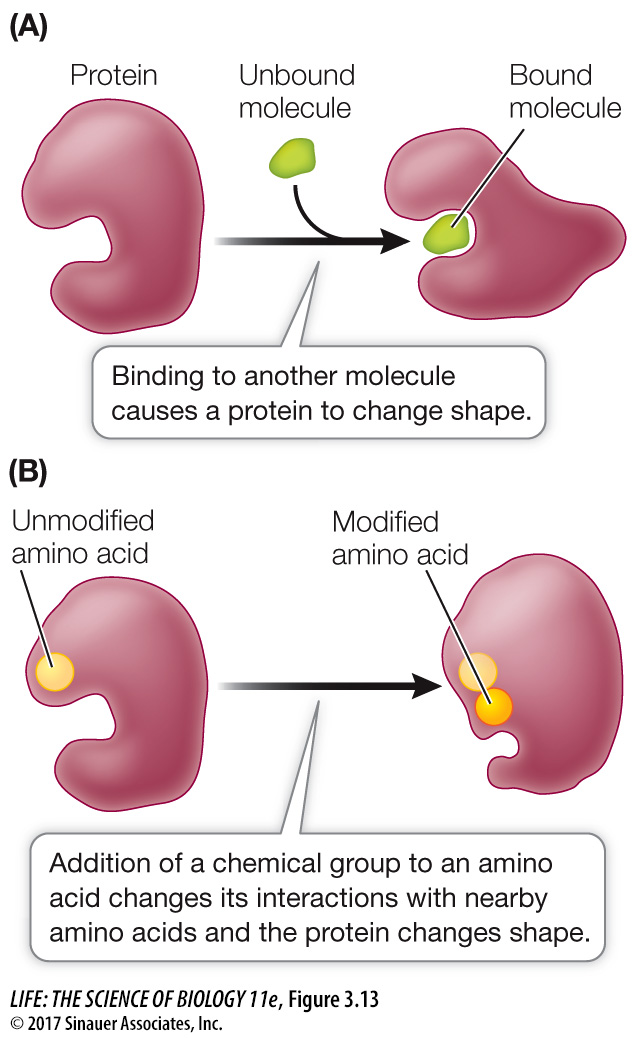 Figure 3.13 Protein Structure Can Change Proteins can change their tertiary structure when they bind to other molecules (A) or are modified chemically (B).
Figure 3.13 Protein Structure Can Change Proteins can change their tertiary structure when they bind to other molecules (A) or are modified chemically (B).Proteins undergo *covalent modifications. After it is made, the structure of a protein can be modified by the covalent bonding of a chemical group to the side chain of one or more of its amino acids. The chemical modification of just one amino acid can alter the shape and function of a protein. An example is the addition of a charged phosphate group to a relatively nonpolar R group. This can cause the amino acid to become more hydrophilic and to move to the outer surface of the protein, altering the shape of the protein in the region near the amino acid (Figure 3.13B).
*connect the concepts Covalent modifications of proteins with subsequent changes in shape and function underlie many biological processes, ranging from signaling within the cell (see Key Concept 7.3) to the action of plant hormones on growth (see Key Concept 37.2).