Apply What You’ve Learned
Review
3.2
A protein’s tertiary structure describes its three-
3.2
Exposed surface groups on a protein provide both shapes and chemical groups that can interact specifically with other molecules or ions.
Original Paper: Conlon, J. M. 2001. Evolution of the insulin molecule: insights into structure-
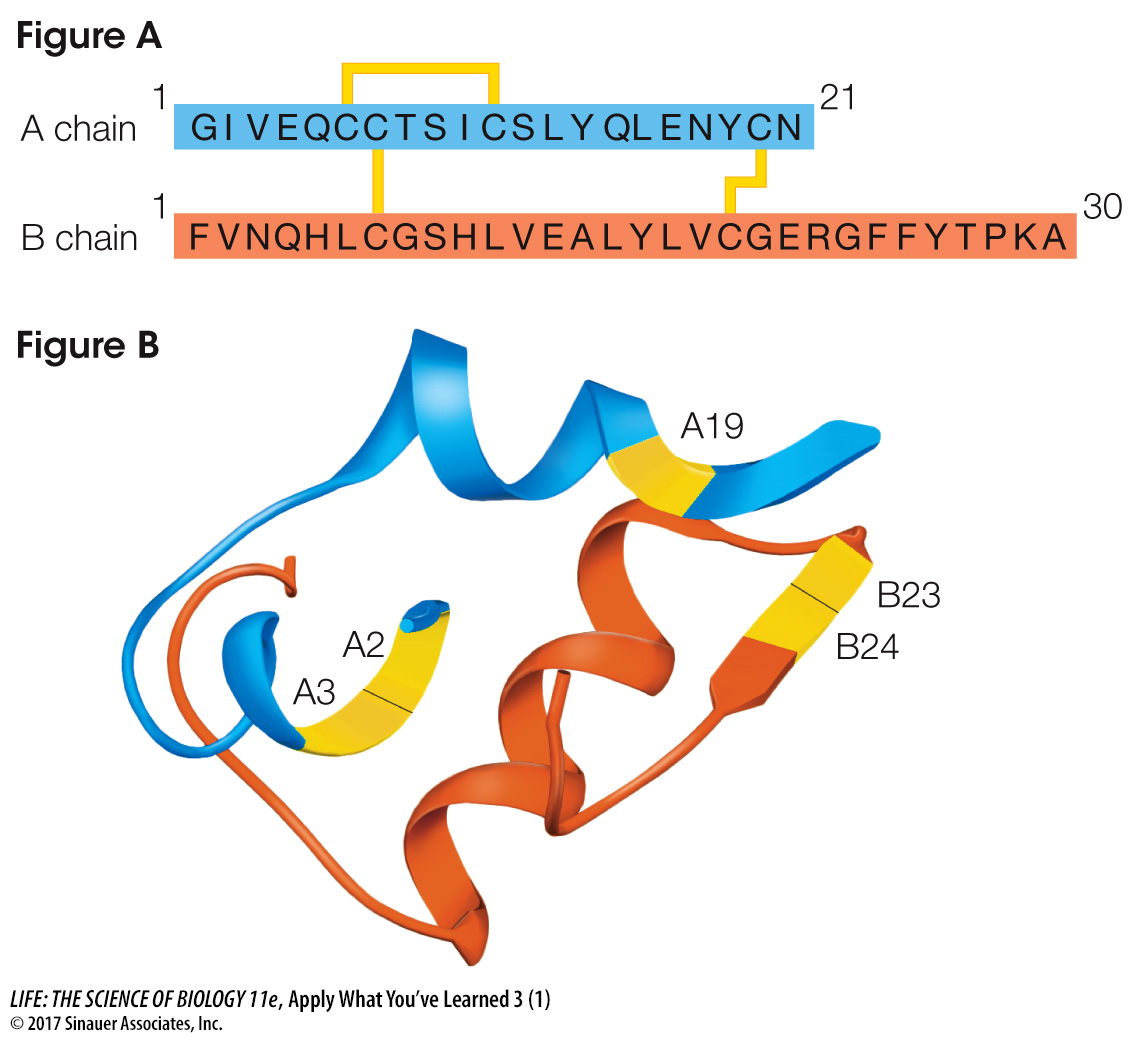
Dogs, like people, tend to have more health problems as they age. About one in every 100 dogs that reach age 12 loses the ability to synthesize insulin, a hormone in the body that regulates cell uptake of glucose from the blood. Veterinarians prescribe injections of porcine insulin for treatment. Porcine insulin is extracted from pigs’ blood and has the same amino acid sequence as canine (dog) insulin. Figure A shows the structure of canine insulin, which is composed of two polypeptides, an A chain and a B chain, linked by disulfide bridges. Figure B shows how these chains fold to form secondary and tertiary structures. Labels A2, A3, A19, B23, and B24 indicate three amino acids on the A chain and two on the B chain thought to be directly involved in the binding of insulin to target proteins on body cell surfaces.
Since insulin is a protein found in all vertebrate animals, scientists have compared the structures of insulin from different vertebrate species. The table shows a comparison of a few amino acids in the A chain and B chain of insulins from a wide range of vertebrates.
| Species | A1 | A2 | A3 | A4 | A5 | A19 | A21 | B12 | B16 | B23 | B24 | B25 | B26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canine | Gly | Ile | Val | Glu | Gln | Tyr | Asn | Val | Tyr | Gly | Phe | Phe | Tyr |
| Guinea pig | — | — | — | Asp | — | — | — | — | — | — | — | — | — |
| Casiragua | — | — | — | Asp | — | — | — | — | — | — | — | Tyr | Arg |
| Porcupine | — | — | — | Asp | — | — | — | — | — | — | — | — | — |
| Cuis | — | — | — | Asp | — | — | — | — | — | — | — | — | Ser |
| Iguana | — | — | — | Gln | — | — | — | — | — | — | — | Tyr | — |
| Rattlesnake | — | — | — | — | — | — | — | — | Phe | — | — | Tyr | — |
| Caecilian | — | — | — | — | Lys | — | — | — | — | — | — | — | — |
| Wood frog | — | — | — | — | — | — | Ser | — | — | — | — | — | — |
| Surinam toad | — | — | — | — | — | — | — | — | His | — | — | — | — |
| Cod | — | — | — | Asp | — | — | — | — | — | — | — | — | — |
| Eel | — | — | — | — | — | — | — | — | — | — | — | — | Phe |
| Tilapia | — | — | — | — | Glu | — | — | — | — | — | — | — | — |
| Bowfin | — | — | — | — | — | — | — | — | Phe | — | — | — | — |
| Spiny dogfish | — | — | — | — | His | — | — | — | — | — | — | Tyr | — |
| Hammerhead shark | — | — | — | Asp | His | — | — | — | — | — | — | Tyr | — |
| Striped gudgeon fish | — | — | — | — | Lys | — | — | — | — | — | — | Tyr | — |
Source: Conlon, J. M. 2001. Evolution of the insulin molecule: Insights into structure-
Note: A dash indicates the amino acid is the same as in the canine.
Questions
1.
What types of forces are involved in the binding of insulin to its target? What would be true about the positions of the amino acids A2, A3, A19, B23, and B24 that would allow them to be involved in binding?
The forces involved are noncovalent and involve mainly hydrophobic and van der Waals interactions, since the R groups tend to be hydrophobic groups (Val, Ile, Phe, Gly). The R groups of these amino acids could stick out from the insulin polypeptide backbone so as to interact with its target proteins to form these noncovalent interactions.
2.
What evidence shown in the table supports the hypothesis that amino acids A2, A3, A19, B23, and B24 are important in insulin’s binding activity?
These amino acids are invariant across all vertebrate species shown in the table, suggesting that they are essential in maintaining insulin’s biological activity.
3.
What conclusion can you draw about the finding that there are amino acid variations in some positions across vertebrate species? What could account for the finding that only certain amino acids are found at those positions and not at a wide range of amino acids?
Certain changes are allowed as long as the overall tertiary structure of the protein is not changed significantly. Only amino acids that are similar in structure and properties would be able to function in a similar way at any position.
4.
Develop a hypothesis about the importance of cysteine residues in canine insulin. How could you test your hypothesis?
Cysteine residues are likely to be essential because they form disulfide bridges, linking the two chains together and stabilizing the protein’s tertiary structure. No other amino acid can fulfill this role. We could compare these amino acids across the same vertebrate species shown above to see whether any have other amino acids at the cysteine locations.