recap
3.1 recap
The four kinds of large molecules that distinguish living tissues are proteins, lipids, carbohydrates, and nucleic acids. Most are polymers: chains of linked monomers. Very large polymers are called macromolecules. Biological molecules carry out a variety of life-
learning outcomes
You should be able to:
Distinguish between structural, cis-
trans , and optical isomers.Give an example of two molecules that have the same functional groups but different properties, and explain the reason for the differences.
Sketch the chemical structures of two biological monomers, and show how a condensation reaction produces a covalent bond between the two.
1.
What are the differences between structural, cis-
Structural isomers have the same number of atoms, but they are arranged differently. Cis-
2.
Examine the isomers mannose and galactose below. What makes them isomers of each other? Which functional groups do these carbohydrates contain, and what properties do these functional groups give to the molecules?
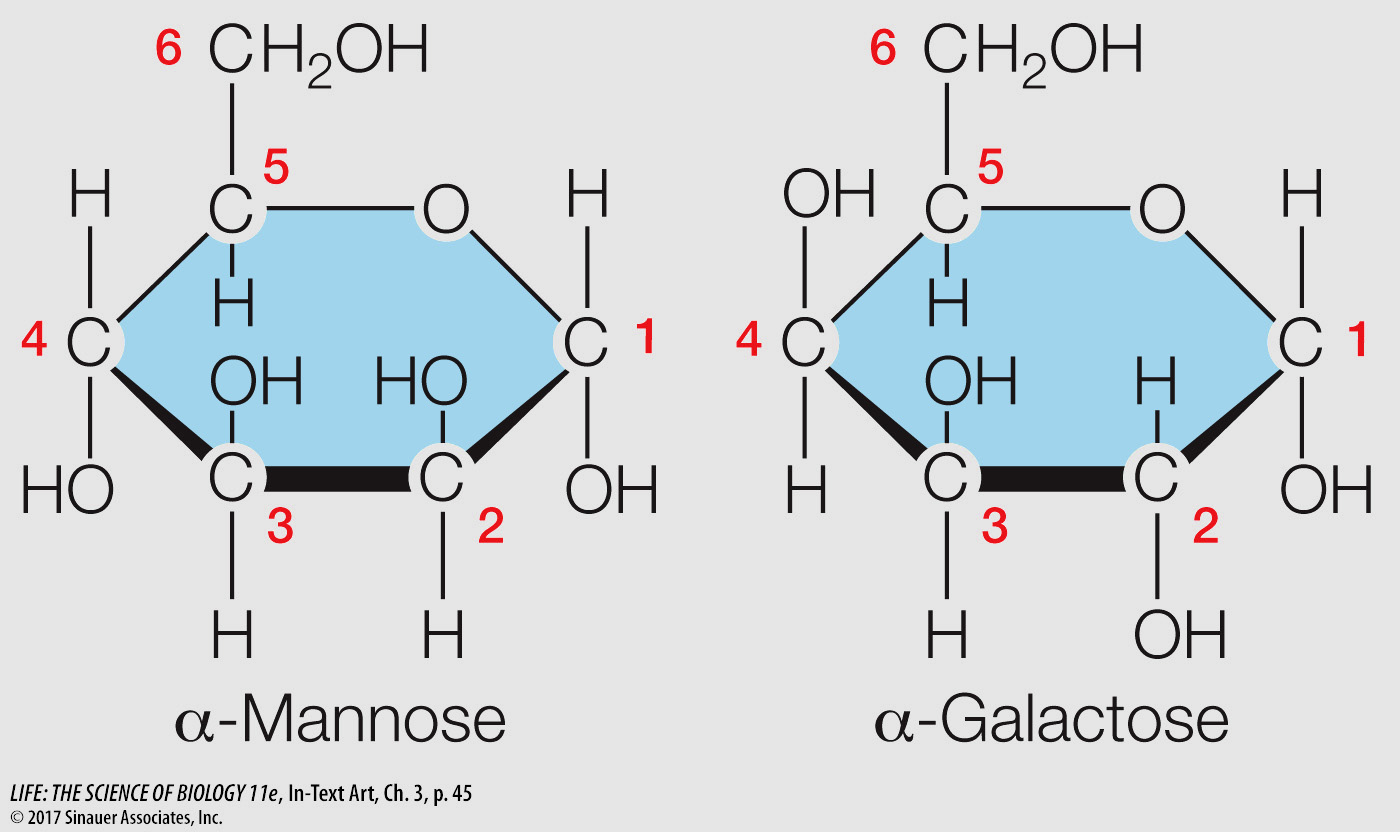
Mannose and galactose have the same atomic formula, C6H12O6, but the arrangement of atoms is different. Compare carbons 2 and 4. These sugars have the hydroxyl (–OH) functional group. Its polarity helps the sugars dissolve in water. The –OH group also can participate in bonding the sugar to other molecules through condensation reactions (see Figures 3.4 and 3.17).
3.
Below are the general structures of three monomers in a biological polymer where A, B, and C are numerous atoms. Draw the reaction that links them together to form a polymer ABC.
H—
H-
H-