Monomers of proteins link together to make the macromolecule
Each amino acid has both a carboxyl functional group and an amino functional group (see Figure 3.1) attached to the same carbon atom, called the α (alpha) carbon. Also attached to the α carbon atom are a hydrogen atom and a side chain, or R group, designated by the letter R.
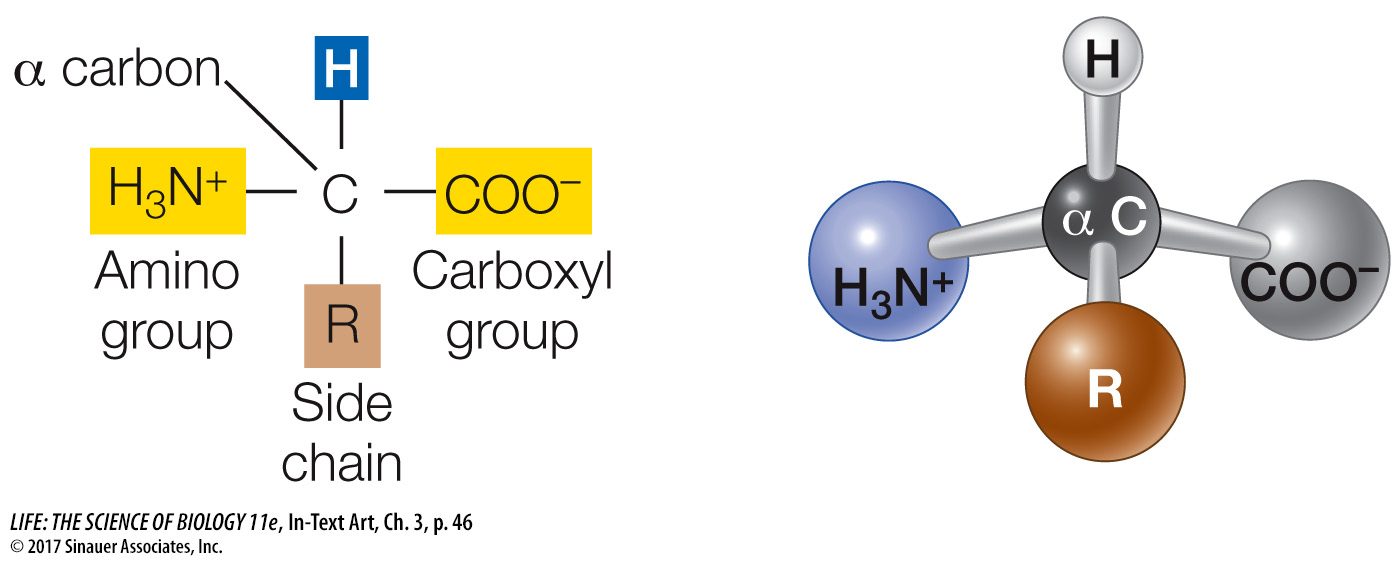
The α carbon in this example is asymmetrical because it is bonded to four different atoms or groups of atoms. Therefore amino acids can exist as optical isomers called D-amino acids and L-amino acids. D and L are abbreviations of the Latin terms for right (dextro) and left (levo). Only L-amino acids (with the configuration shown above) are commonly found in the proteins of most organisms, and their presence is an important chemical “signature” of life.
At the pH levels typically found in cells (usually about pH 7), both the carboxyl and amino groups of amino acids are ionized: the carboxyl group has lost a hydrogen ion:
—COOH → —COO– + H+
and the amino group has gained a hydrogen ion:
—NH2 + H+ → —NH3+
Thus amino acids are simultaneously acids and bases.
Activity 3.2 Features of Amino Acids
The side chains (or R groups) of amino acids contain functional groups that are important in determining the three-
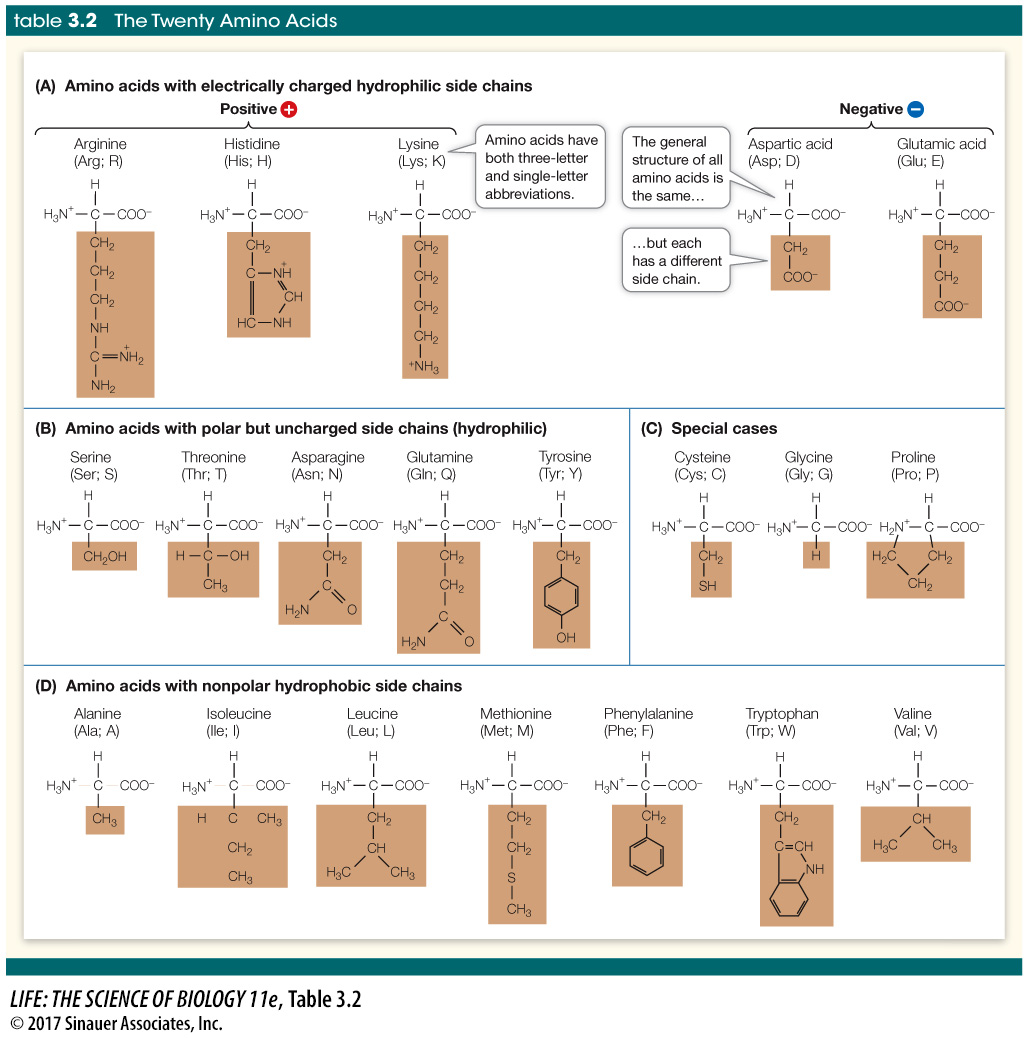
Five amino acids have electrically charged (ionized) side chains at pH levels typical of living cells. These side chains attract water (are hydrophilic) and attract oppositely charged ions of all sorts.
Five amino acids have polar side chains. They are also hydrophilic and attract other polar or charged molecules.
Seven amino acids have side chains that are nonpolar and thus hydrophobic. In the watery environment of the cell, these hydrophobic groups may cluster together in the interior of the protein.
Three amino acids—
The cysteine side chain, which has a terminal —SH group, can react with another cysteine side chain in an oxidation reaction to form a covalent bond (Figure 3.5). Such a bond, called a disulfide bridge or disulfide bond (—S—
S— ), helps determine how a polypeptide chain folds. 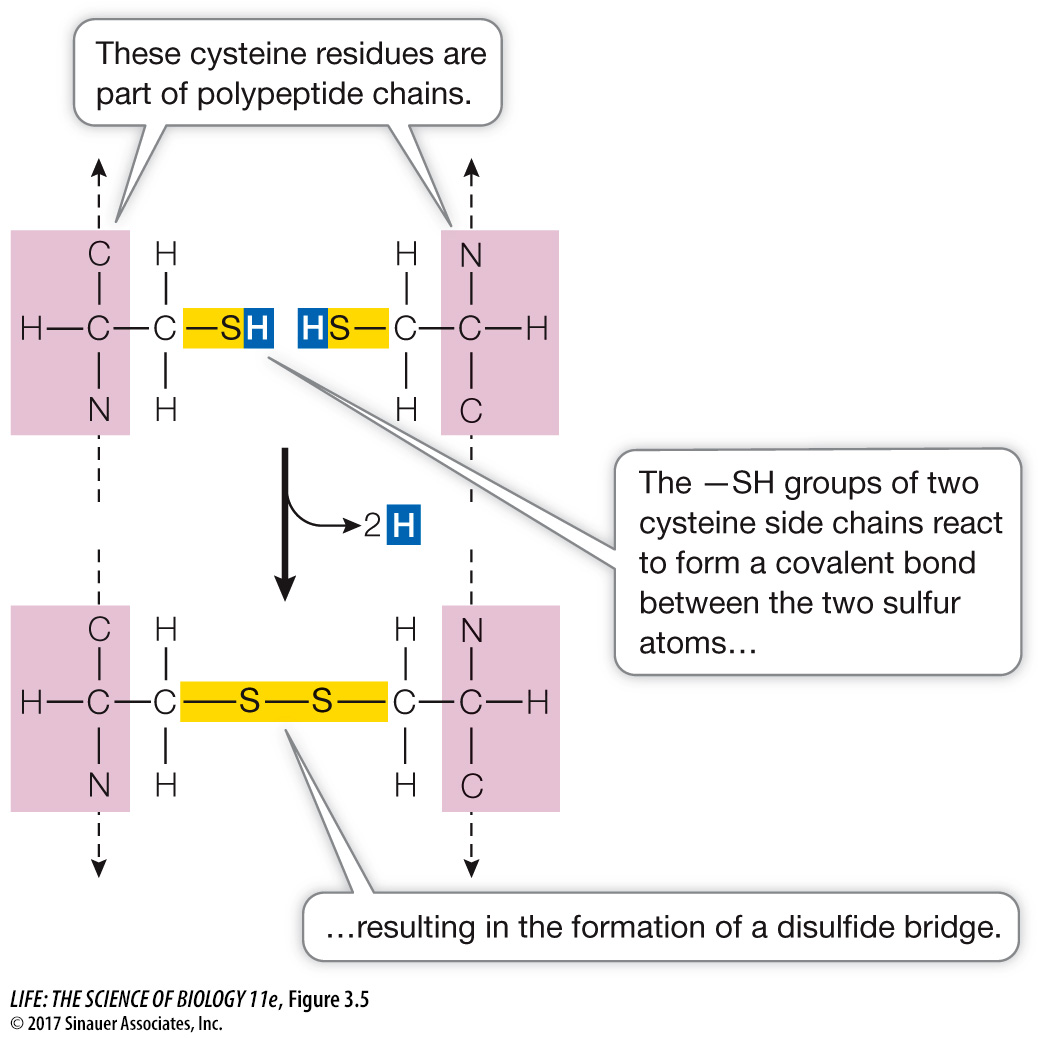 Figure 3.5 A Disulfide Bridge Two cysteine molecules in a polypeptide chain can form a disulfide bridge (—S—
Figure 3.5 A Disulfide Bridge Two cysteine molecules in a polypeptide chain can form a disulfide bridge (—S—S— ) by oxidation (removal of H atoms). Page 47The glycine side chain consists of a single hydrogen atom. It is small enough to fit into tight corners in the interiors of protein molecules where larger side chains could not fit.
Proline possesses a modified amino group that lacks a hydrogen atom and instead forms a covalent bond with the hydrocarbon side chain, resulting in a ring structure. This limits both its hydrogen-
bonding ability and its ability to rotate around the α carbon. So proline is often found where a protein bends or loops.