Peptide linkages form the backbone of a protein
Linking amino acids involves a reaction between carboxyl and amino groups attached to the α carbon. The carboxyl group of one amino acid reacts with the amino group of another, undergoing a condensation reaction that forms a peptide linkage (also called a peptide bond). Figure 3.6 depicts this reaction.
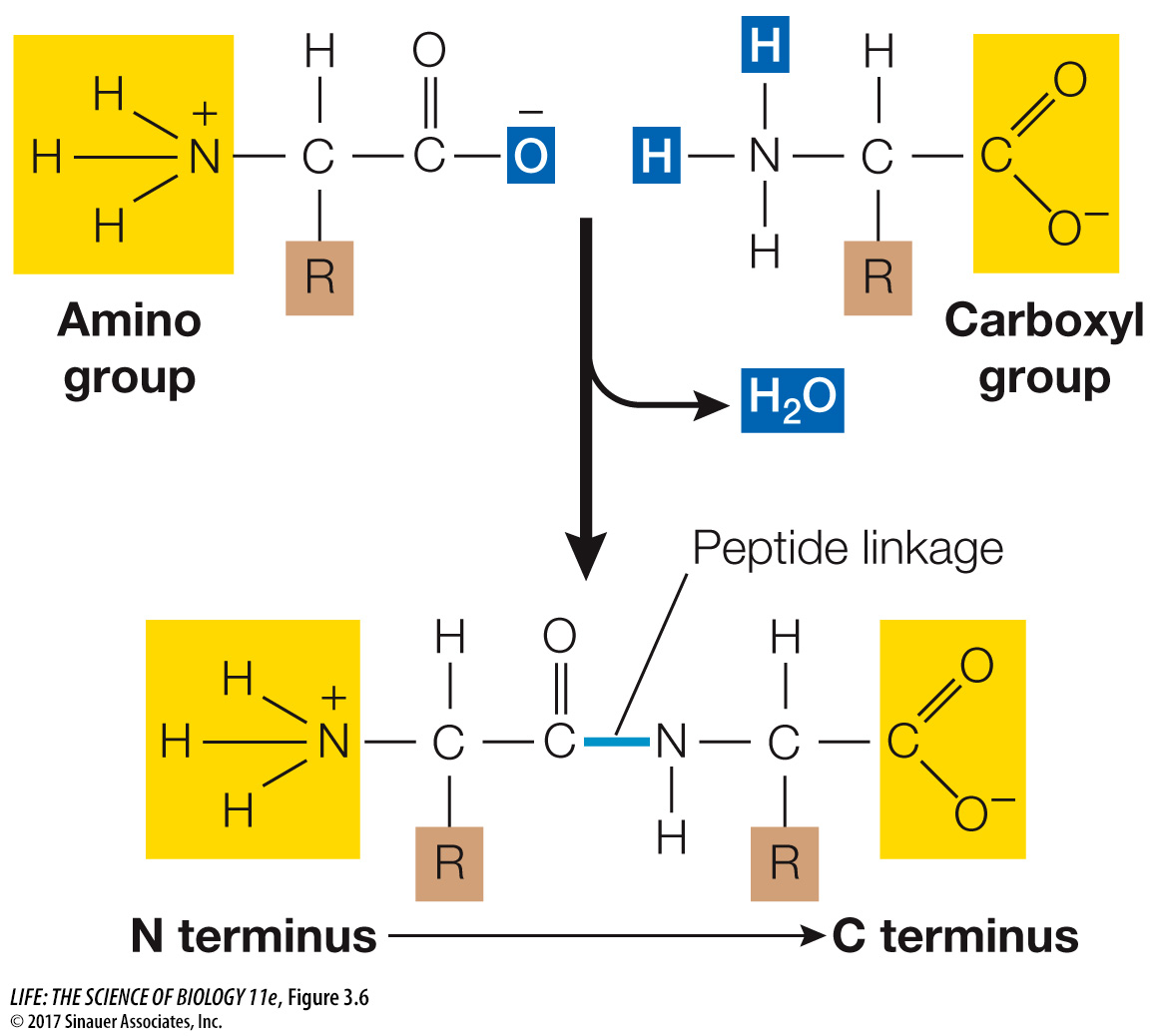
Just as a sentence begins with a capital letter and ends with a period, polypeptide chains have a beginning and an end. The “capital letter” marking the beginning of a polypeptide is the amino group of the first amino acid added to the chain and is known as the N terminus. The “period” is the carboxyl group of the last amino acid added; this is the C terminus.
Two characteristics of the peptide bond are especially important in the three-
In the C—
N linkage, the adjacent α carbons (α C— C— N— α C) are not free to rotate fully, which limits the folding of the polypeptide chain. The oxygen bound to the carbon (C=O) in the carboxyl group carries a slight negative charge (δ–), whereas the hydrogen bound to the nitrogen (N—
H) in the amino group is slightly positive (δ+). This asymmetry of charge favors hydrogen bonding within the protein molecule itself and between molecules. These bonds contribute to the structures and functions of many proteins.
In addition to these characteristics of the peptide linkage, the particular sequence of amino acids—
focus: key figure
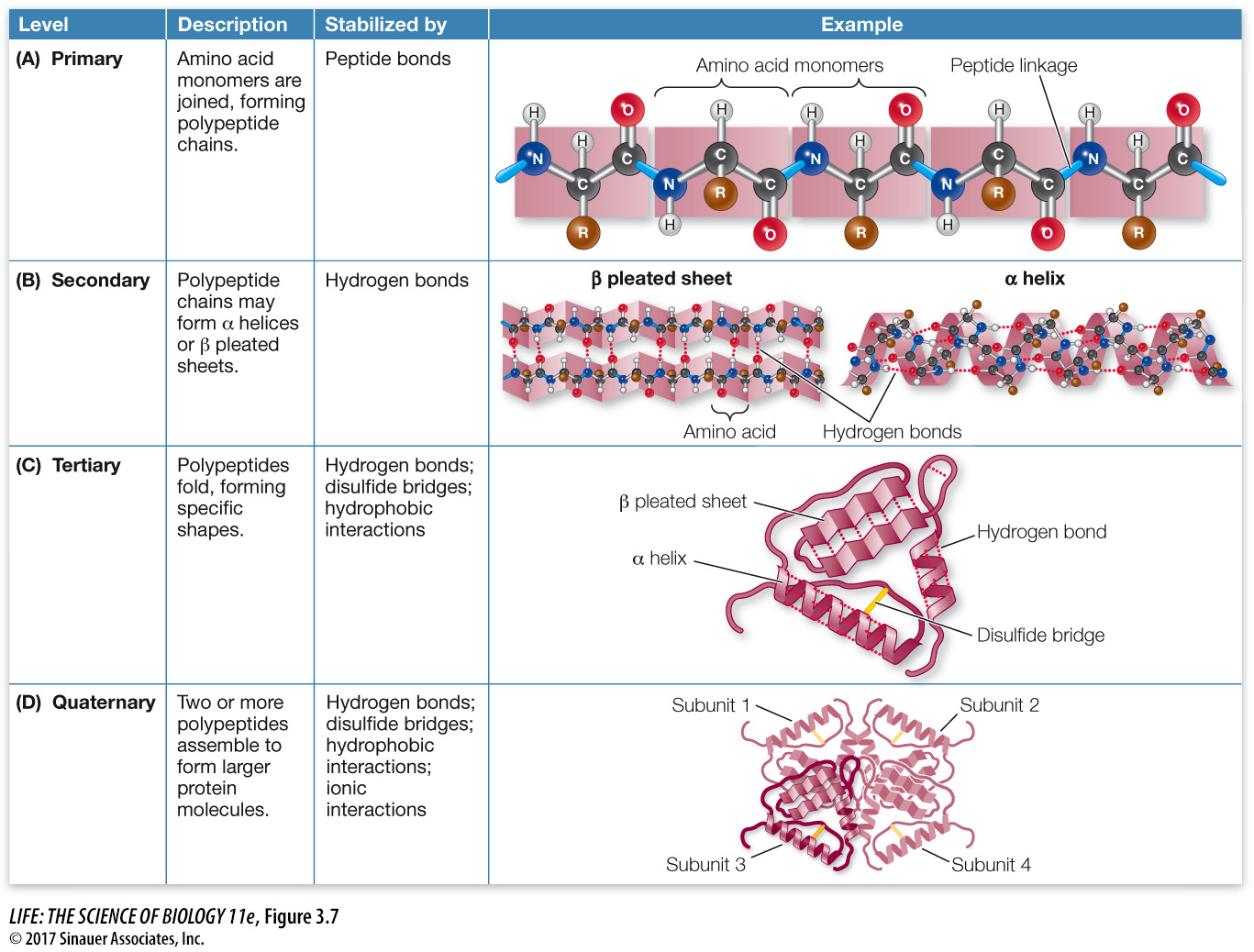
Q: If a protein is gently heated to break hydrogen bonds, what level(s) of structure will be unaffected, and why?
Primary structure will be unaffected, because it is held together by strong covalent bonds.