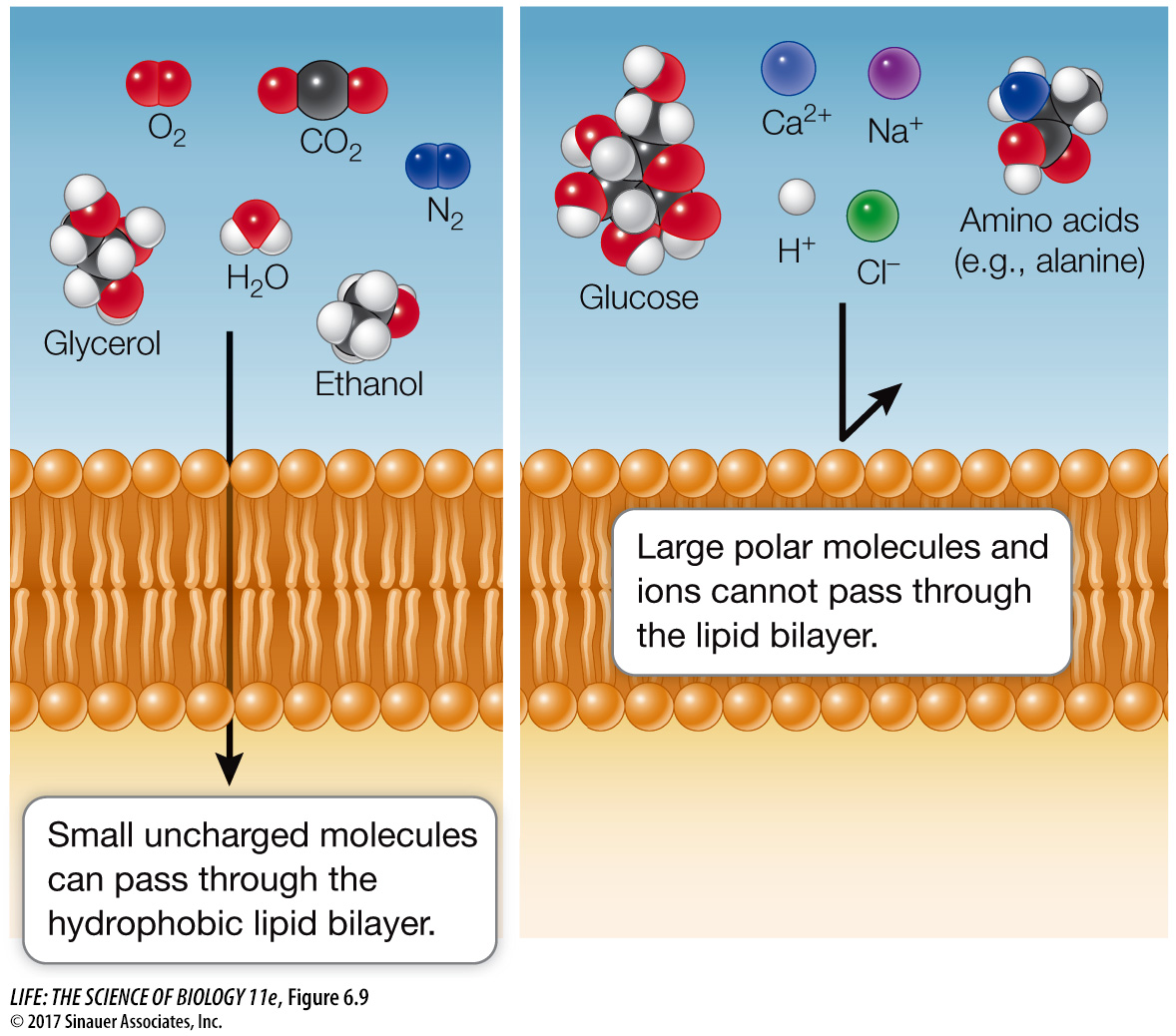
Simple diffusion takes place through the phospholipid bilayer
In simple diffusion, small molecules pass through the phospholipid bilayer of the membrane. A molecule that is itself hydrophobic, and is therefore soluble in lipids, enters the membrane readily and is able to pass through it. The more lipid-
By contrast, electrically charged or polar molecules, such as amino acids, sugars, and ions, do not pass readily through a membrane, for two reasons. First, such charged or polar molecules are not very soluble in the hydrophobic interior of the bilayer. Second, such substances form many hydrogen bonds with water and ions in the aqueous environment, be it the cytoplasm or the cell exterior. The multiplicity of these hydrogen bonds prevents the substances from moving into the hydrophobic interior of the membrane. Figure 6.9 summarizes these phenomena.