Membrane proteins are asymmetrically distributed
All biological membranes contain proteins. Typically, cell membranes have 1 protein molecule for every 25 lipid molecules. This ratio varies depending on membrane function. In the inner membrane of the mitochondrion, which is specialized for energy processing, there is 1 protein for every 15 lipids. However, myelin—
Membrane proteins are very diverse. In fact, about one-
Integral membrane proteins are at least partly embedded in the phospholipid bilayer (see Figure 6.1). Like phospholipids, these proteins have both hydrophilic and hydrophobic regions (domains) (Figure 6.3).
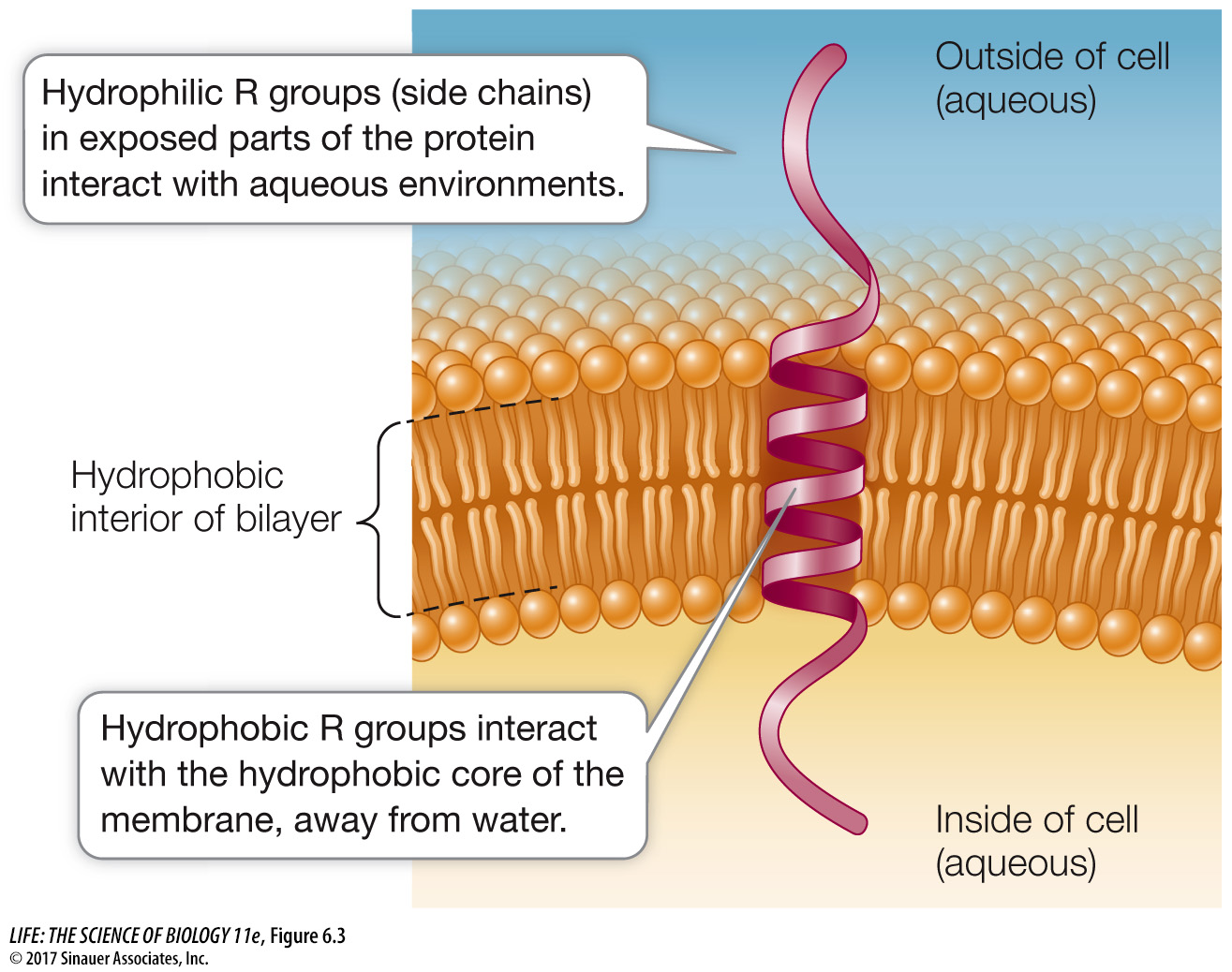
Hydrophilic domains: Stretches of amino acids with hydrophilic side chains (R groups; see Table 3.2) give certain regions of the protein a polar character. These hydrophilic domains interact with water and stick out into the aqueous environment inside or outside the cell.
Hydrophobic domains: Stretches of amino acids with hydrophobic side chains give other regions of the protein a nonpolar character. These domains interact with the fatty acids in the interior of the phospholipid bilayer, away from water. Some proteins contain covalently linked lipids such as fatty acid chains that do the job of inserting into the membrane (instead of the protein having hydrophobic amino acid regions).
Peripheral membrane proteins lack exposed hydrophobic groups and are not embedded in the bilayer. Instead, they have polar or charged regions that interact with exposed parts of integral membrane proteins, or with the polar heads of phospholipid molecules (see Figure 6.1).
A special preparation method for electron microscopy, called freeze-
research tools
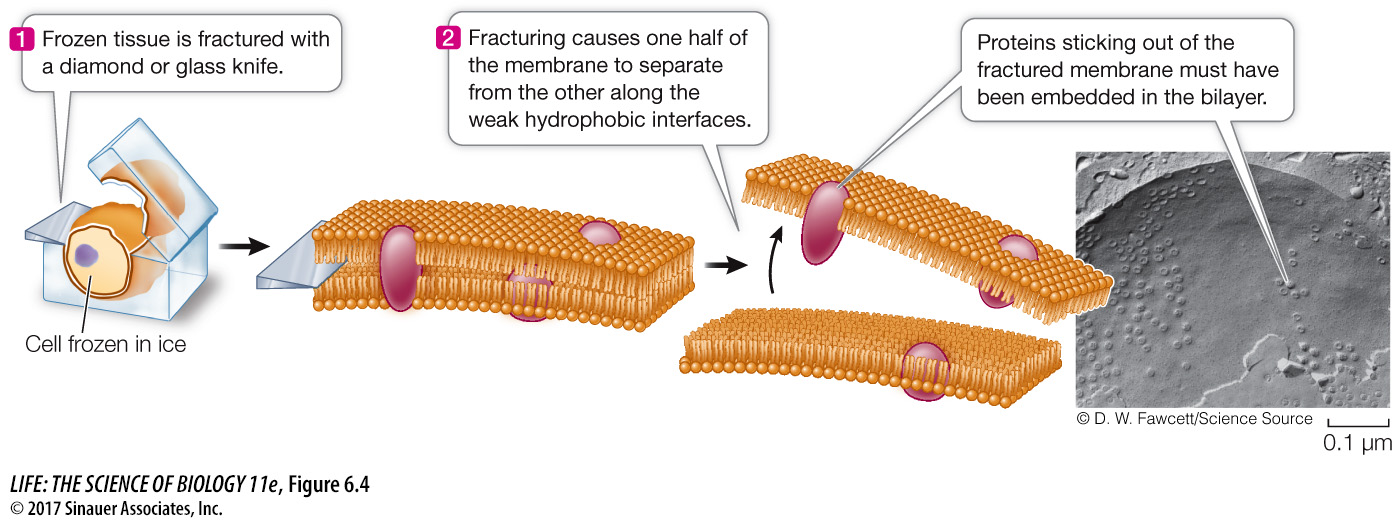
Membrane proteins and lipids generally interact only noncovalently. The polar ends of proteins can interact with the polar ends of lipids, and the nonpolar regions of both molecules can interact hydrophobically. As mentioned above, however, some membrane proteins have hydrophobic lipid components covalently attached to them that allow these proteins to tether themselves to the phospholipid bilayer.
Proteins are asymmetrically distributed on the inner and outer surfaces of membranes. An integral protein that extends all the way through the phospholipid bilayer and protrudes on both sides is known as a transmembrane protein. In addition to one or more transmembrane domains that extend through the bilayer, such a protein may have domains with other specific functions on the inner and outer sides of the membrane. Peripheral membrane proteins, by contrast, are located on one side of the membrane or the other. This asymmetrical arrangement of membrane proteins gives the two surfaces of the membrane different properties. As you will soon see, these differences have great functional significance.
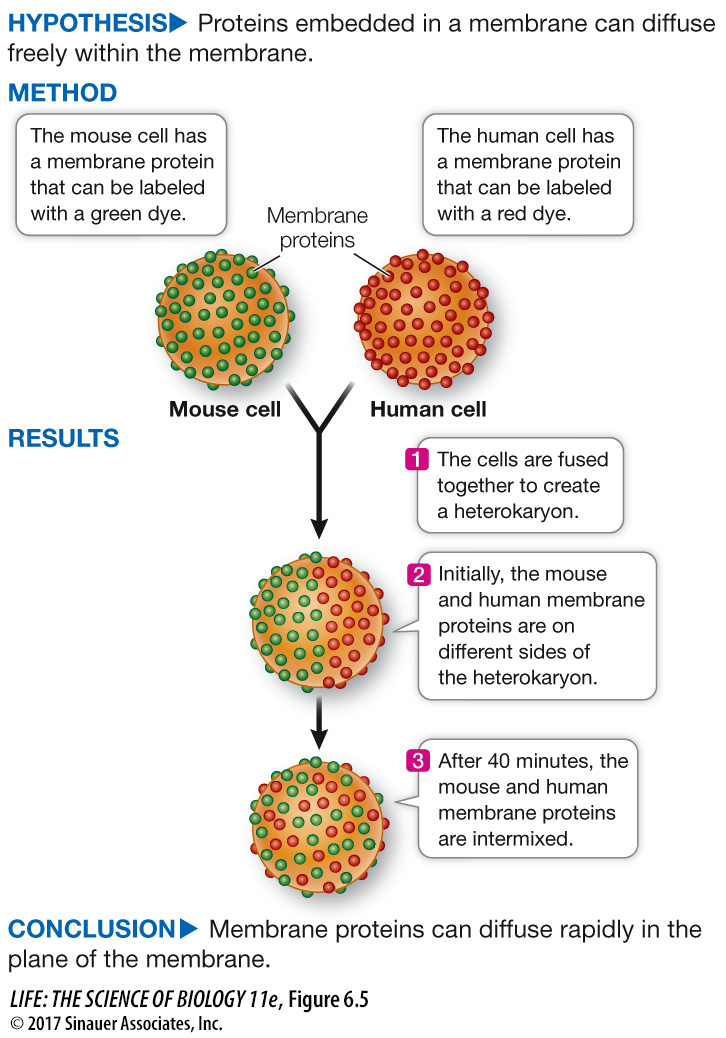
Like lipids, some membrane proteins move around relatively freely within the phospholipid bilayer. Experiments that involve the technique of cell fusion illustrate this migration dramatically. When two cells are fused, a single continuous membrane forms and surrounds both cells, and some proteins from each cell distribute themselves uniformly around this membrane (Figure 6.5).
experiment
Original Paper: Frye, L. D. and M. Edidin. 1970. The rapid intermixing of cell surface antigens after formation of mouse-
Two animal cells can be fused together in the laboratory, forming a single large cell (heterokaryon). This phenomenon was used to test whether membrane proteins can diffuse independently in the plane of the cell membrane.
Although some proteins are free to migrate in the membrane, others are not, but rather appear to be “anchored” to a specific region of the membrane. These membrane regions are like an enclosure of animals at the zoo: the animals are free to move around within the fenced area but not outside it. An example is the protein in the cell membrane of a muscle cell that recognizes a chemical signal from a neuron. This protein is normally found only at the specific region where the neuron meets the muscle cell. Proteins inside the cell can restrict the movement of proteins within a membrane. The cytoskeleton has components just below the inner face of the membrane that are attached to membrane proteins protruding into the cytoplasm. The stability of the cytoskeletal components may thus restrict movement of attached membrane proteins.