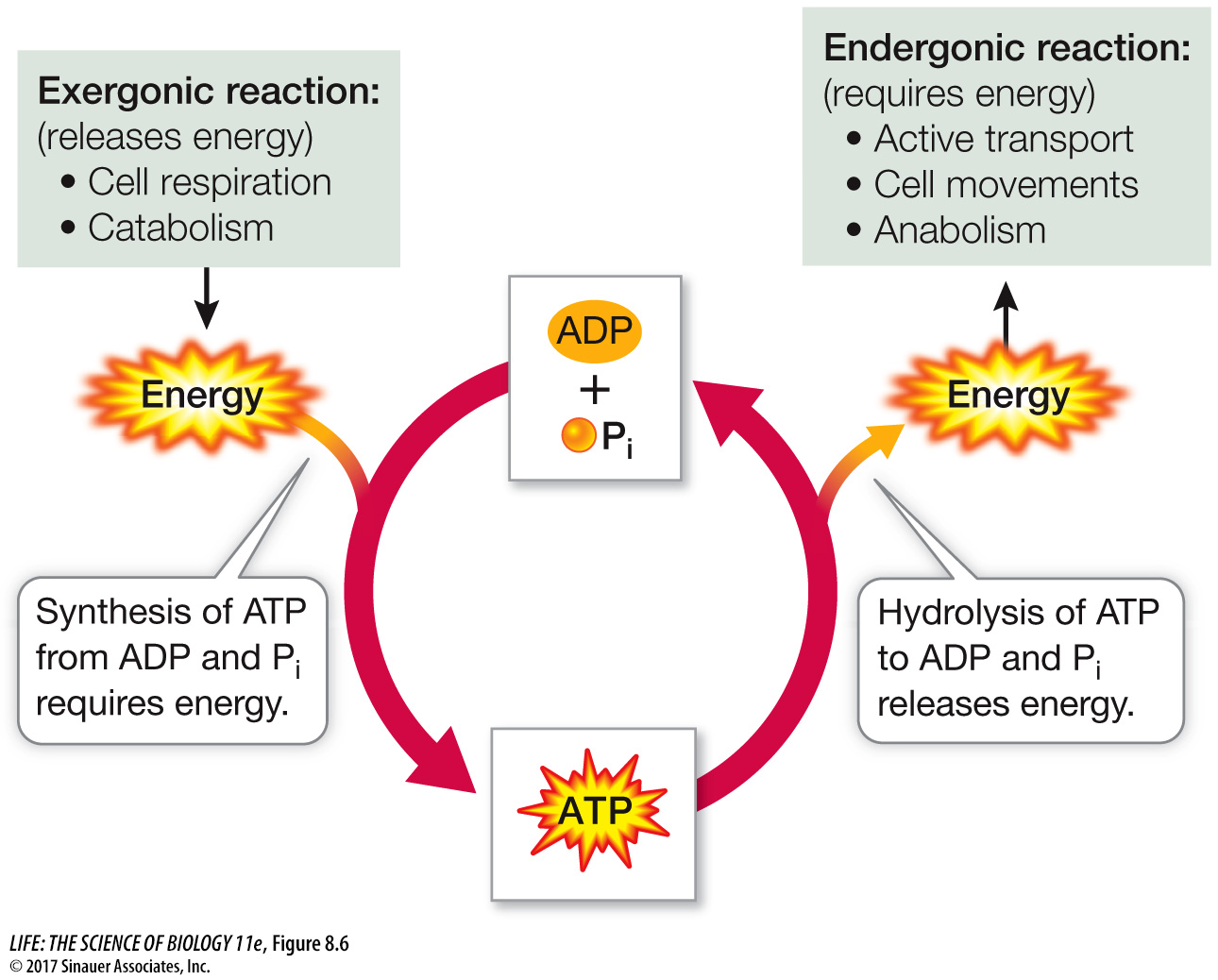
ATP couples exergonic and endergonic reactions
As you have just seen, the hydrolysis of ATP is exergonic and yields ADP, Pi, and more free energy (or AMP, PPi, and more free energy). The reverse reaction, the formation of ATP from ADP and Pi, is endergonic and consumes as much free energy as is released by the hydrolysis of ATP:
ADP + Pi + free energy → ATP + H2O
Many exergonic reactions in the cell can provide the energy to convert ADP into ATP. For eukaryotes and many prokaryotes, the most important of these reactions is cellular respiration, in which some of the energy released from fuel molecules is captured in ATP. The formation and hydrolysis of ATP constitute what might be called an “energy-
Coupling of exergonic and endergonic reactions is very common in metabolism. Free energy is captured and retained in the P~O bonds of ATP. ATP then diffuses to another place in the cell, where its hydrolysis releases the free energy to drive an endergonic reaction. For example, the formation of glucose 6-
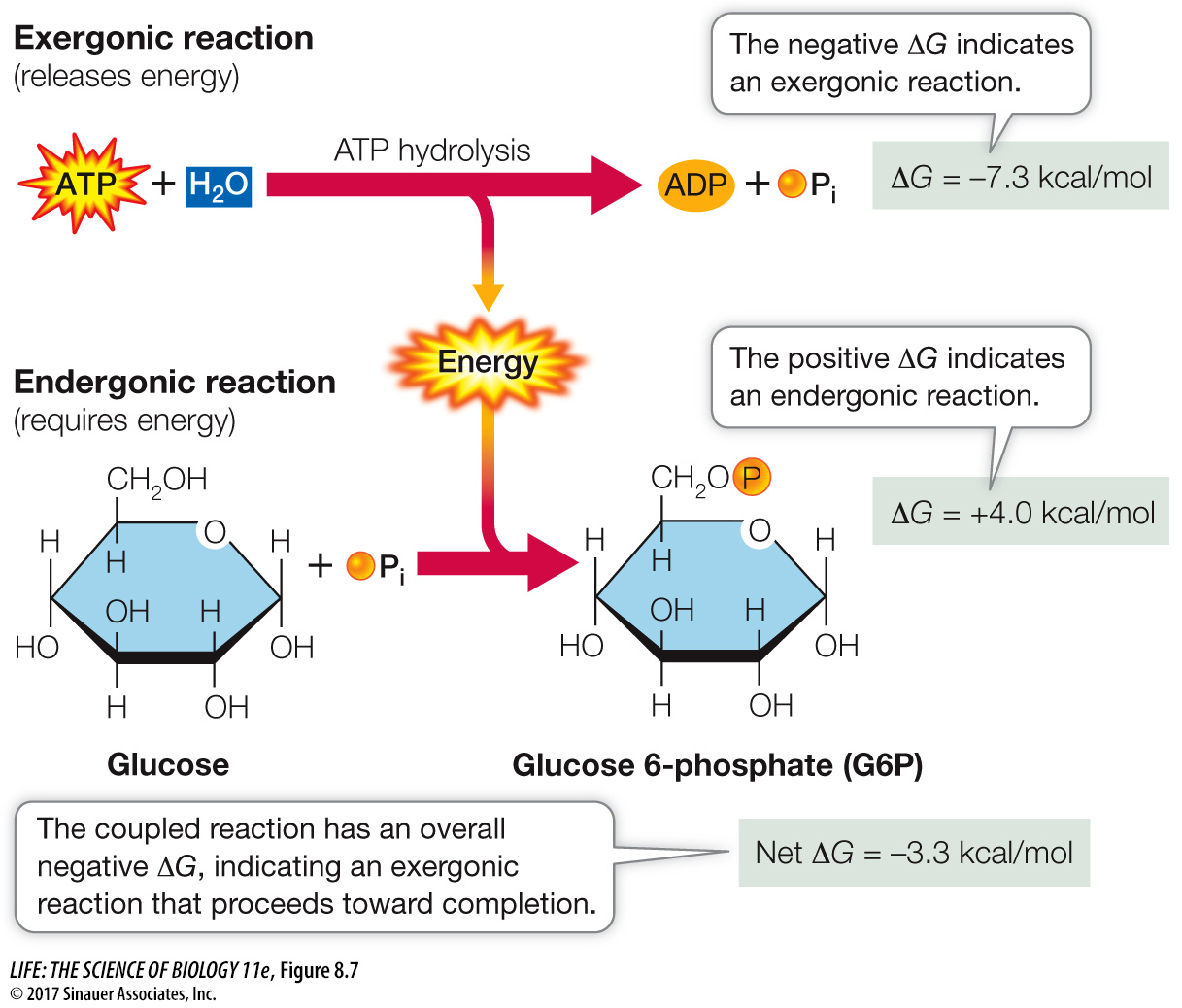
Q: What would be the magnitude of the ΔG of a different conversion that could drive the synthesis of ATP?
The synthesis of ATP has a ΔG of +7.3 kcal/mol. So any reaction driving this synthesis must have a ΔG of at least –7.3 kcal/mol. Because of the second law of thermodynamics, the transfer of energy is not efficient (entropy increases), so the ΔG of the driving reaction would be more than +7.3 kcal/mol.
An active cell requires the production of millions of molecules of ATP per second to drive its biochemical machinery. From previous chapters, you may already be familiar with some of the activities in the cell that require energy from the hydrolysis of ATP:
Active transport across a membrane (see Figure 6.14)
Condensation reactions that use enzymes to form polymers (see Figure 3.4A)
Modifications of cell signaling proteins by protein kinases (see Figure 7.15)
Motor proteins that move vesicles along microtubules (see Figure 5.19)
An ATP molecule is typically consumed within a second of its formation. At rest, an average person produces and hydrolyzes about 40 kg of ATP per day—