Enzymes lower the energy barrier but do not affect equilibrium
Reactants that are bound to the enzyme, forming an enzyme–substrate complex, require less activation energy than do the transition-state intermediates in the corresponding uncatalyzed reaction (Figure 8.10). Thus the enzyme lowers the energy barrier for the reaction—it offers the reaction an easier path, speeding it up. When an enzyme lowers the energy barrier, both the forward and the reverse reactions speed up, so the enzyme-catalyzed reaction proceeds toward equilibrium more rapidly than the uncatalyzed reaction. The final equilibrium is the same with or without the enzyme. Similarly, adding an enzyme to a reaction does not change the difference in free energy (ΔG) between the reactants and the products (see Figure 8.10).
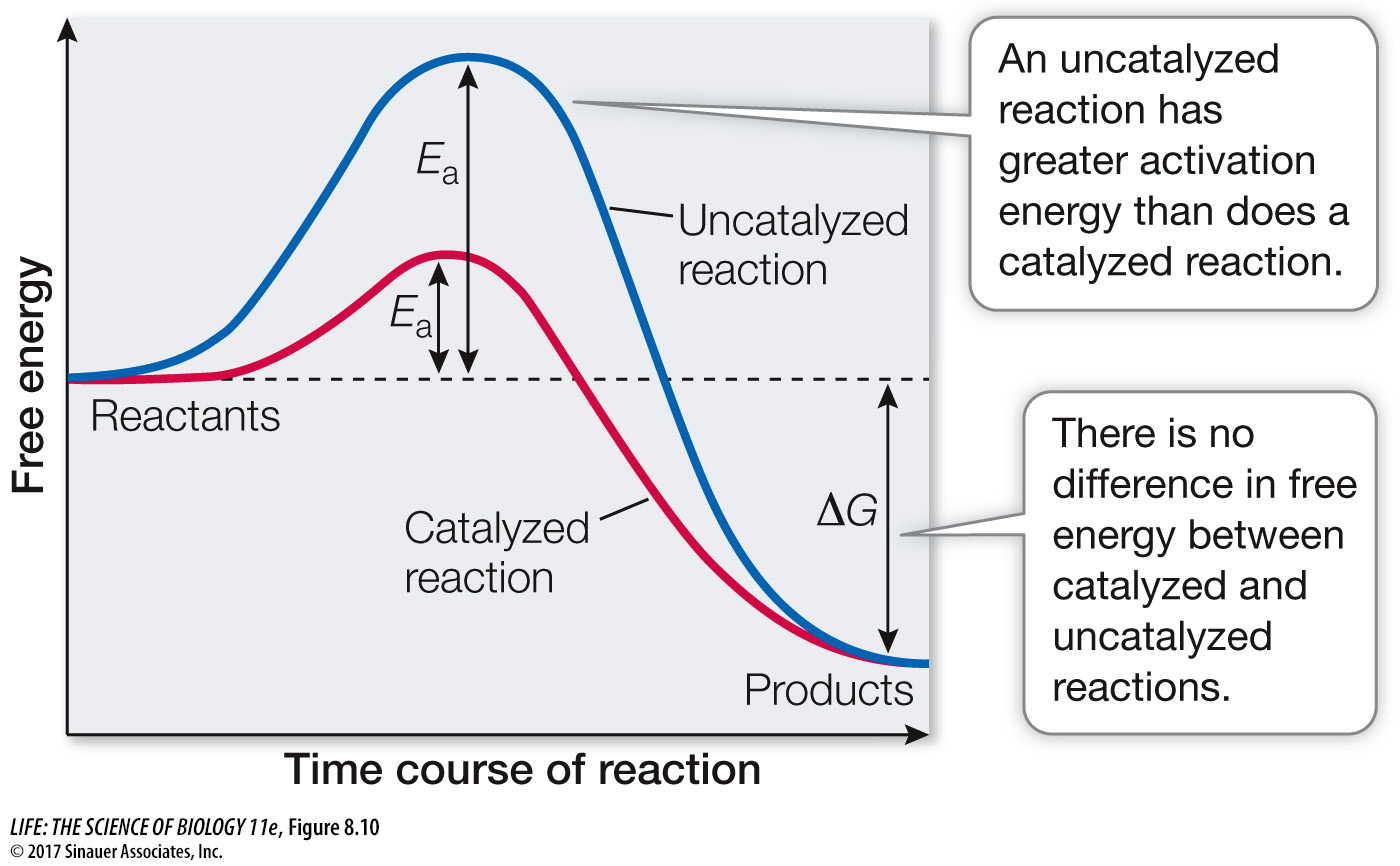
Figure 8.10 Enzymes Lower the Energy Barrier Although the activation energy is lower in an enzyme-catalyzed reaction than in an uncatalyzed reaction, the energy released is the same with or without catalysis. In other words, Ea is lower but ΔG is unchanged. Lower activation energy means the reaction will take place at a faster rate.
Page 160
Activity 8.2 Free Energy Changes
Enzymes can change the rate of a reaction substantially. For example, if a particular protein that has arginine as its terminal amino acid just sits in solution, the protein molecules tend toward disorder and the terminal peptide bonds break, releasing the arginine residues (ΔS increases). Without an enzyme, this is a very slow reaction—it takes about 7 years for half of the protein molecules to undergo the reaction. But, with the enzyme carboxypeptidase A catalyzing the reaction, half the arginines are released in less than a second! Rate enhancement by enzymes varies from 1 million times to an amazing 1026 by sulfate monoesterases. The consequence of catalysis for living cells is not difficult to imagine. Such increased reaction rates make new realities possible.
