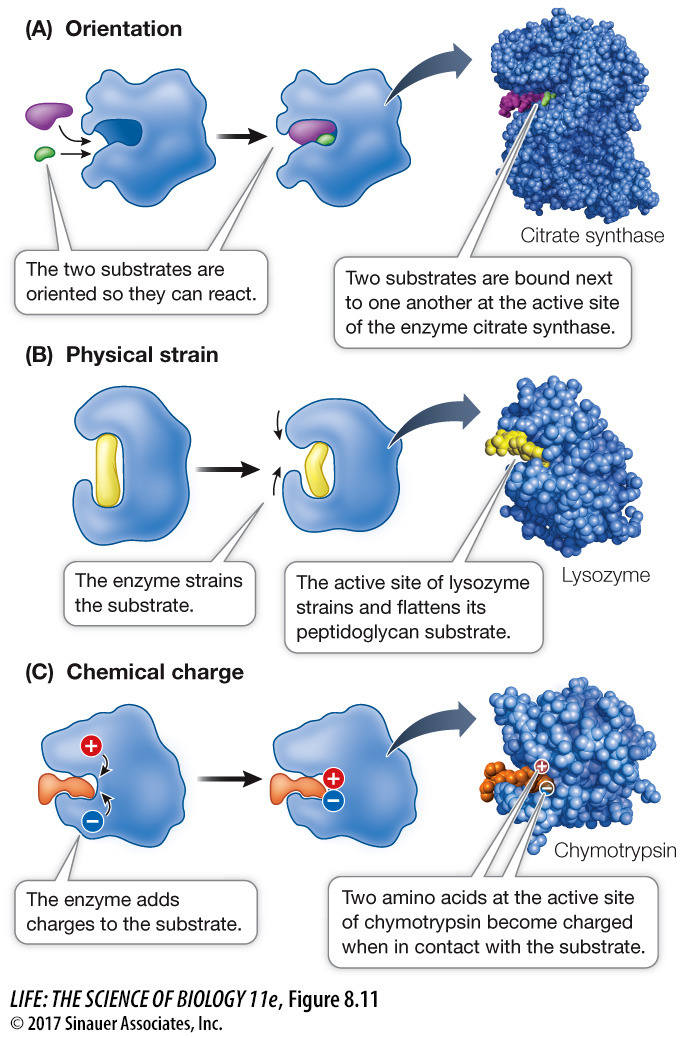
Enzymes can orient substrates
When free in solution, substrates are moving from place to place randomly while at the same time vibrating, rotating, and tumbling around. They may not have the proper orientation to interact when they collide. Part of the activation energy needed to start a reaction is used to bring together specific atoms so that bonds can form (Figure 8.11A). For example, if acetyl CoA and oxaloacetate are to form citrate (a step in the metabolism of glucose; see Key Concept 9.2), the two substrates must be oriented so that the carbon atom of the methyl group of acetyl CoA can form a covalent bond with the carbon atom of the carbonyl group of oxaloacetate. The active site of the enzyme citrate synthase has just the right shape to bind these two molecules so that these atoms are adjacent.