Chemical equilibrium and free energy are related
Every chemical reaction proceeds to a certain extent, but not necessarily to completion (all reactants converted into products). Each reaction has a specific equilibrium point, which is related to the relative free energy content of the reactants and products. To understand the principle of equilibrium, let’s consider the following example, which takes place inside most cells but which we can do in the lab. This is the interconversion of glucose 1-
Glucose 1-
Imagine that we start out with an aqueous solution of glucose 1-
At equilibrium, then, this reaction has a product-
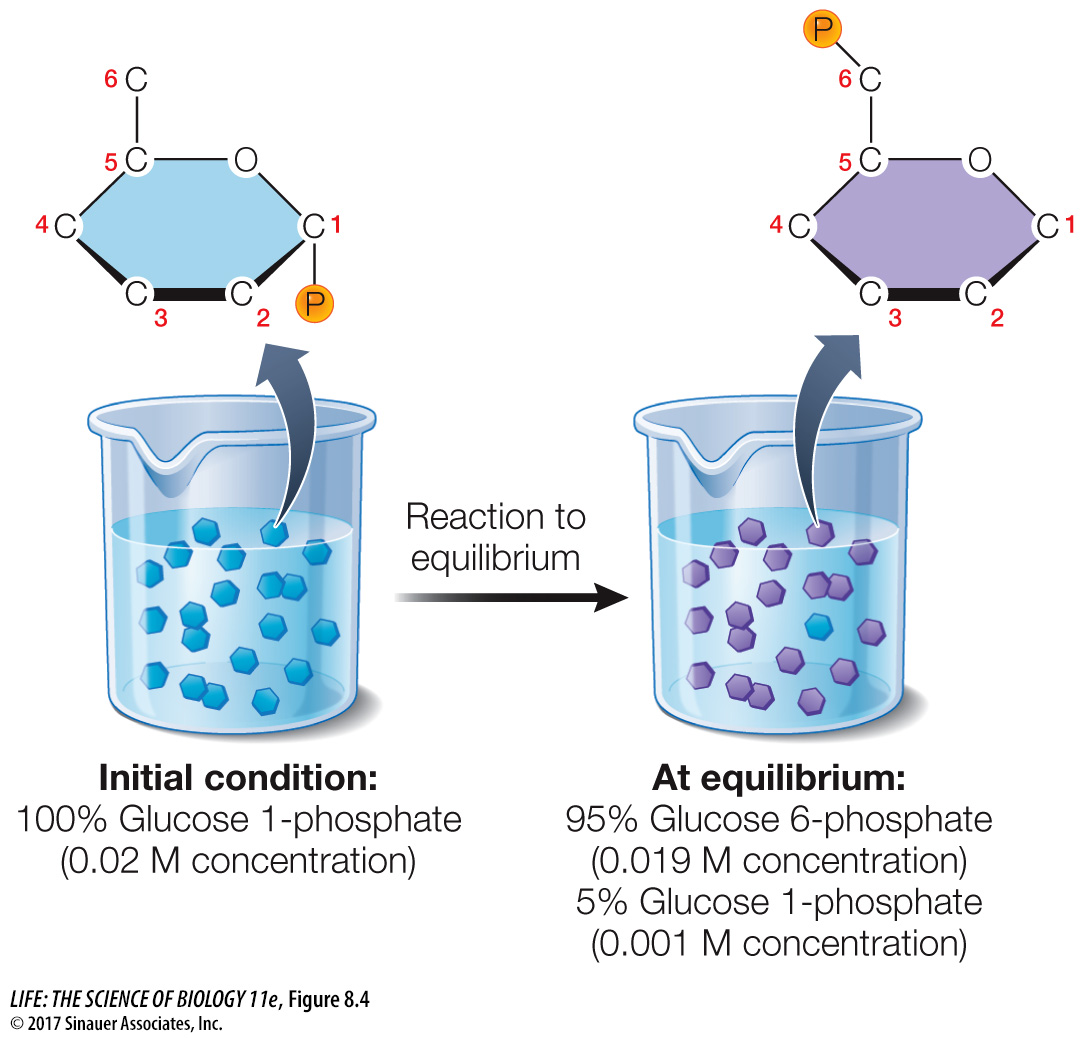
The change in free energy (ΔG) for any reaction is related directly to its point of equilibrium. The further toward completion the point of equilibrium lies, the more free energy is released. In an exergonic reaction, ΔG is a negative number. The total value of ΔG also depends on the beginning concentrations of the reactants and products and other conditions such as temperature, pressure, and pH of the solution. Biochemists often calculate ΔG using standard laboratory conditions: 25°C, one atmosphere pressure, one molar (1 M) concentrations of the solutes, and pH 7. The standard free energy change calculated using these conditions is designated ΔG0 In our example of the conversion of glucose 1-
A large, positive ΔG for a reaction means that it does not proceed to the right (A → B). If the concentration of B is initially high relative to that of A, such a reaction runs “to the left” (A ← B), and at equilibrium nearly all of B is converted into A. A ΔG value near zero is characteristic of a readily reversible reaction: reactants and products have almost the same free energies.
In Chapters 9 and 10 you will learn the biochemical conversions that extract energy from food and light. In turn, this energy is used to synthesize carbohydrates, lipids, and proteins. All of the chemical reactions carried out by living organisms are governed by the principles of thermodynamics and equilibrium.