Printed Page 178(cont.)
Pyruvate oxidation and the citric acid cycle are regulated by the concentrations of starting materials
You have now seen that pyruvate, a three-
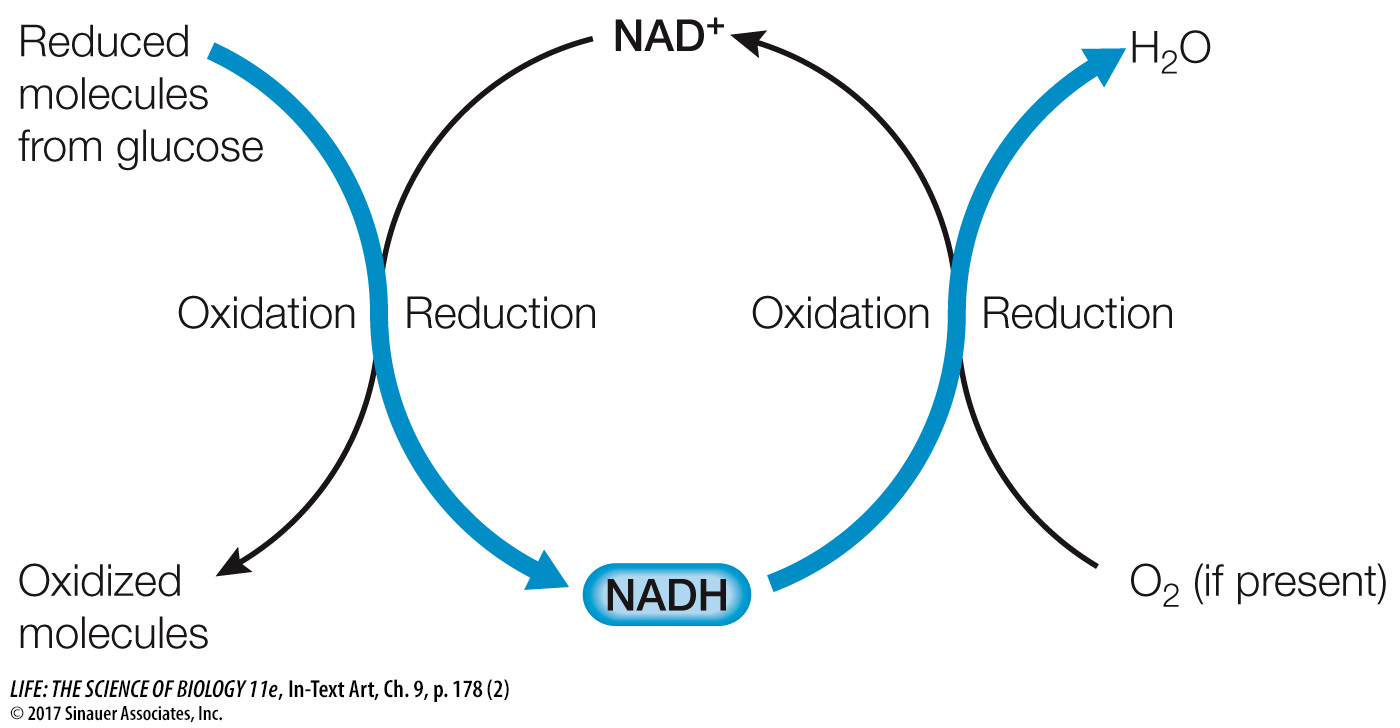
NADH → NAD+ + H+ + 2 e–
FADH2 → FAD + 2 H+ + 2 e–
These oxidation reactions are coupled to reactions in which other molecules get reduced. When it is present, O2 is the molecule that eventually accepts these electrons, and it is reduced to form H2O.