ATP is made through chemiosmosis
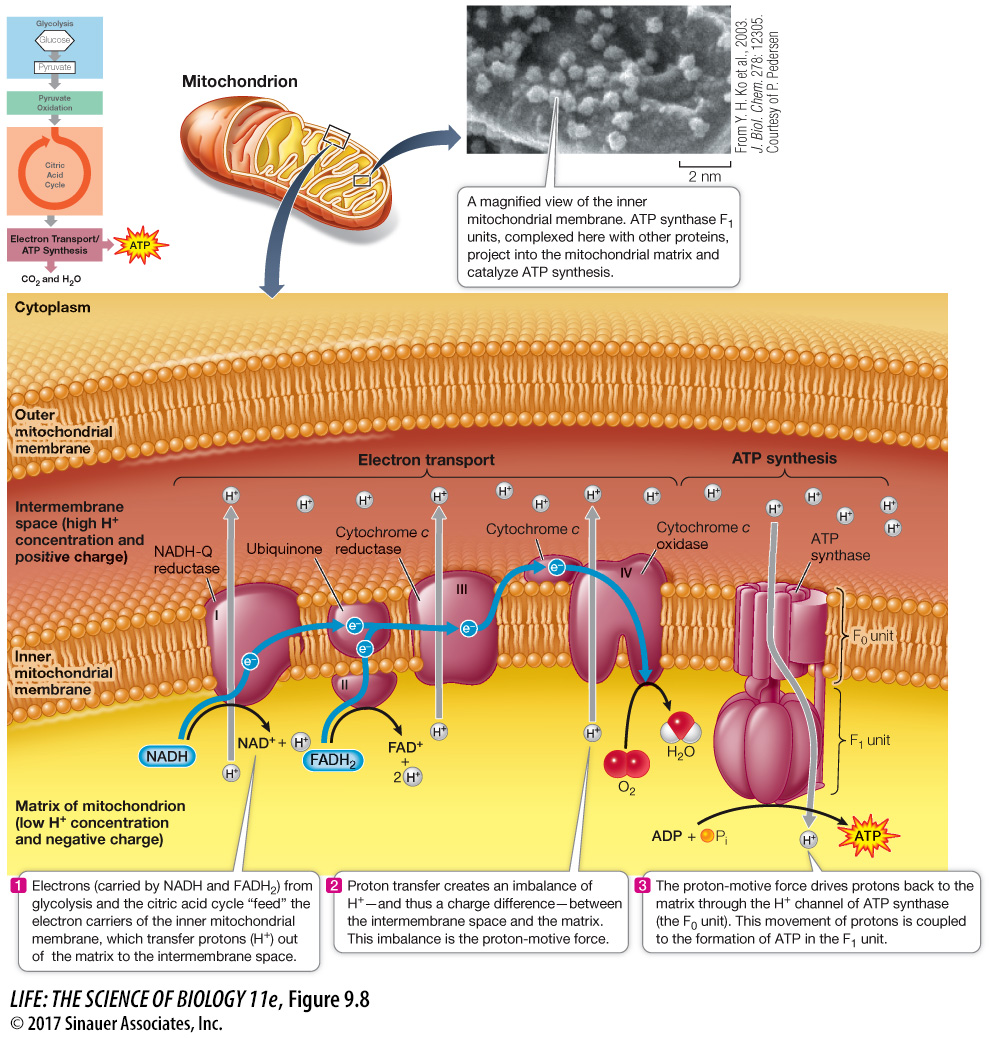
During electron transport, protons are also actively transported across the membrane: electron transport within each of the three transmembrane complexes (I, III, and IV) results in the transfer of protons from the matrix to the intermembrane space (Figure 9.8). Since the lipid bilayer does not allow charged H+ to diffuse across it, transfer of H+ across it through the electron transport chain sets up a gradient, with the concentration of H+ in the intermembrane space higher than in the matrix. In addition, because H+ carries a charge, there is more positive charge in the intermembrane space. These two gradients—
Animation 9.1 Electron Transport and ATP Synthesis
Activity 9.5 Electron Transport Simulation
How can this energy be tapped for use by the cell? The answer is that another protein, ATP synthase, allows the H+ to diffuse back into the matrix down its concentration gradient. In the process, potential energy is captured and used for the formation of ATP. The coupling of the proton-
To summarize, the energy originally contained in glucose and other fuel molecules is ultimately captured in the cellular energy currency, ATP. For each pair of electrons passed along the chain from NADH to oxygen, about 2.5 molecules of ATP are formed. FADH2 oxidation produces about 1.5 ATP molecules because it enters the electron transport chain at a later step than NADH (see Figure 9.8).
ATP synthesis is a reversible reaction, and ATP synthase can also act as an ATPase, hydrolyzing ATP to ADP and Pi:
ATP ⇌ ADP + Pi + free energy
If the reaction goes to the right, free energy is released. In the mitochondrion, it is used to transfer H+ out of the mitochondrial matrix—
ATP leaves the mitochondrial matrix for use elsewhere in the cell as soon as it is made, keeping the ATP concentration in the matrix low, and driving the reaction toward the left.
The H+ gradient is maintained by electron and proton transport.
Every day a person hydrolyzes about 1025 ATP molecules to ADP. This amounts to 9 kg, a significant fraction of the person’s entire body weight! The vast majority of this ADP is “recycled”—converted back to ATP—