recap
9.1 recap
The free energy released from the oxidation of glucose is trapped in the form of ATP. In many prokaryotes and all eukaryotes, five major pathways combine in different ways to produce ATP, which supplies the energy for myriad other reactions in living cells.
learning outcomes
You should be able to:
Describe the principles of chemistry that govern metabolic pathways in cells.
Identify the change in energy that occurs during an oxidation–
reduction reaction. Describe the roles of electron acceptors and donors in biological redox reactions.
Analyze biological redox reactions to identify the oxidizing and reducing agents involved.
1.
What principles govern metabolic pathways in cells?
A complex chemical transformation occurs in a series of separate reactions that form a metabolic pathway. Each reaction is catalyzed by a specific enzyme. Many metabolic pathways are similar in all organisms, from bacteria to humans. In eukaryotes, many metabolic pathways are compartmentalized, with certain reactions occurring inside specific organelles. Some key enzymes in each metabolic pathway can be inhibited or activated to alter the rate of the pathway.
2.
What are the roles of NAD+ and O2 with respect to electrons in a redox reaction?
NAD+ is a reducing agent (it accepts electrons), and O2 is an oxidizing agent (it donates electrons).
3.
The following reaction occurs in the citric acid cycle:
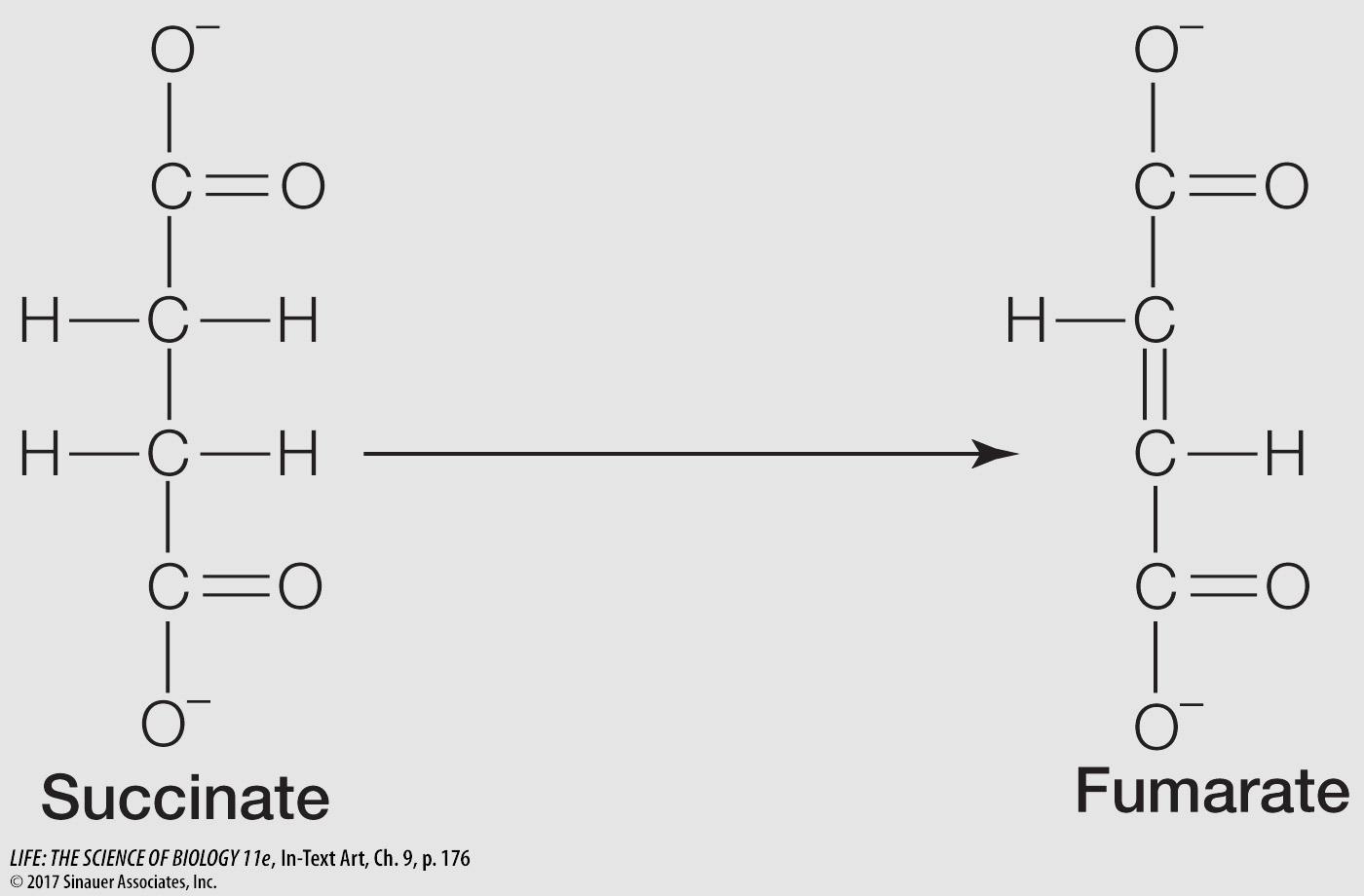
Answer each of the following questions, and explain your answers:
Is this reaction an oxidation or a reduction?
It is an oxidation (removal of H from C2 and C3 of succinate).
Is the reaction exergonic or endergonic?
It is exergonic, because it is an oxidation.
What kind of coenzyme does this reaction require?
It requires the redox coenzyme NAD or FAD.
Now that you have an overview of the metabolic pathways that harvest energy from glucose, let’s take a closer look at the three pathways involved in aerobic glucose catabolism: glycolysis, pyruvate oxidation, and the citric acid cycle.