Light energy is absorbed by pigments in photosynthesis
It is helpful here to discuss light in terms of its photochemistry and photobiology.
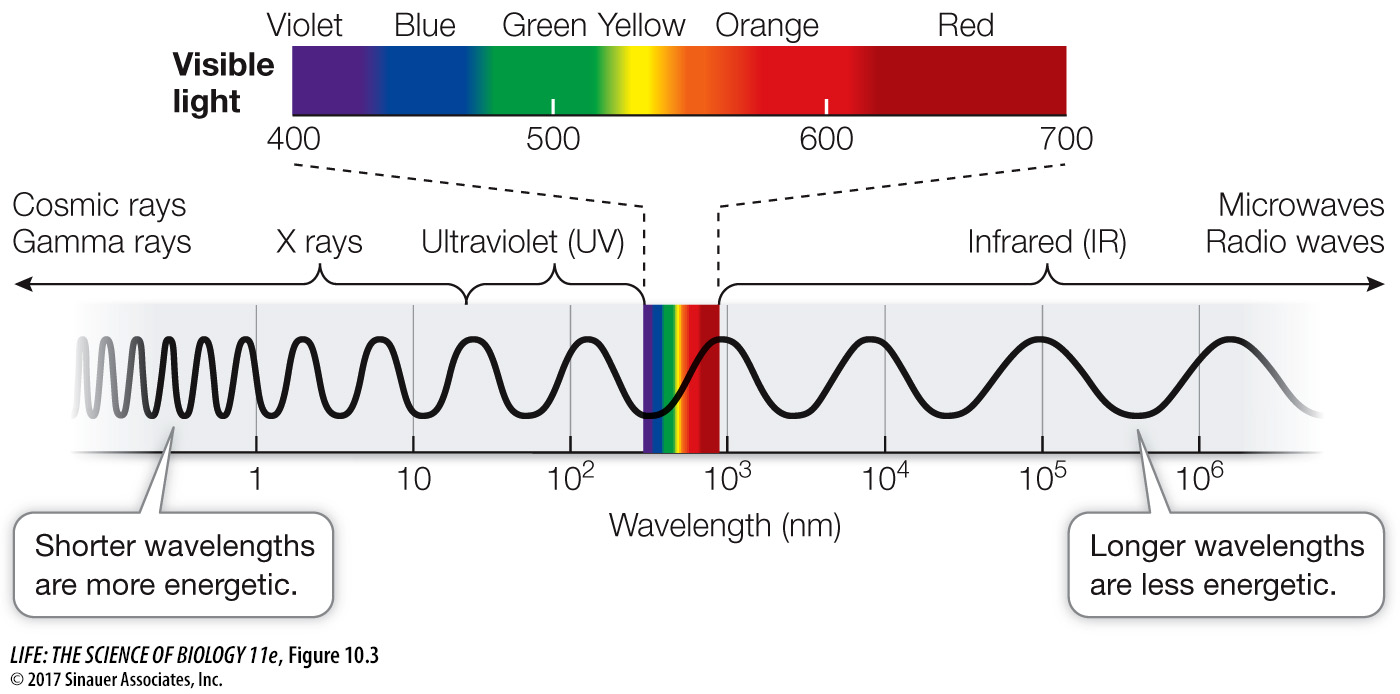
PHOTOCHEMISTRY Light is a form of electromagnetic radiation. Electromagnetic radiation is said to have a dual nature. Although it is propagated in waves, it also has particle-
When a photon meets a molecule, one of three things can happen:
The photon may bounce off the molecule—
it may be scattered or reflected. The photon may pass through the molecule—
it may be transmitted. The photon may be absorbed by the molecule, adding energy to the molecule.
Neither of the first two outcomes causes any change in the molecule. However, in the case of absorption, the photon disappears and its energy is absorbed by the molecule. The photon’s energy cannot disappear, because according to the first law of thermodynamics, energy is neither created nor destroyed. When the molecule acquires the energy of the photon, it is raised from a ground state (with lower energy) to an excited state (with higher energy):
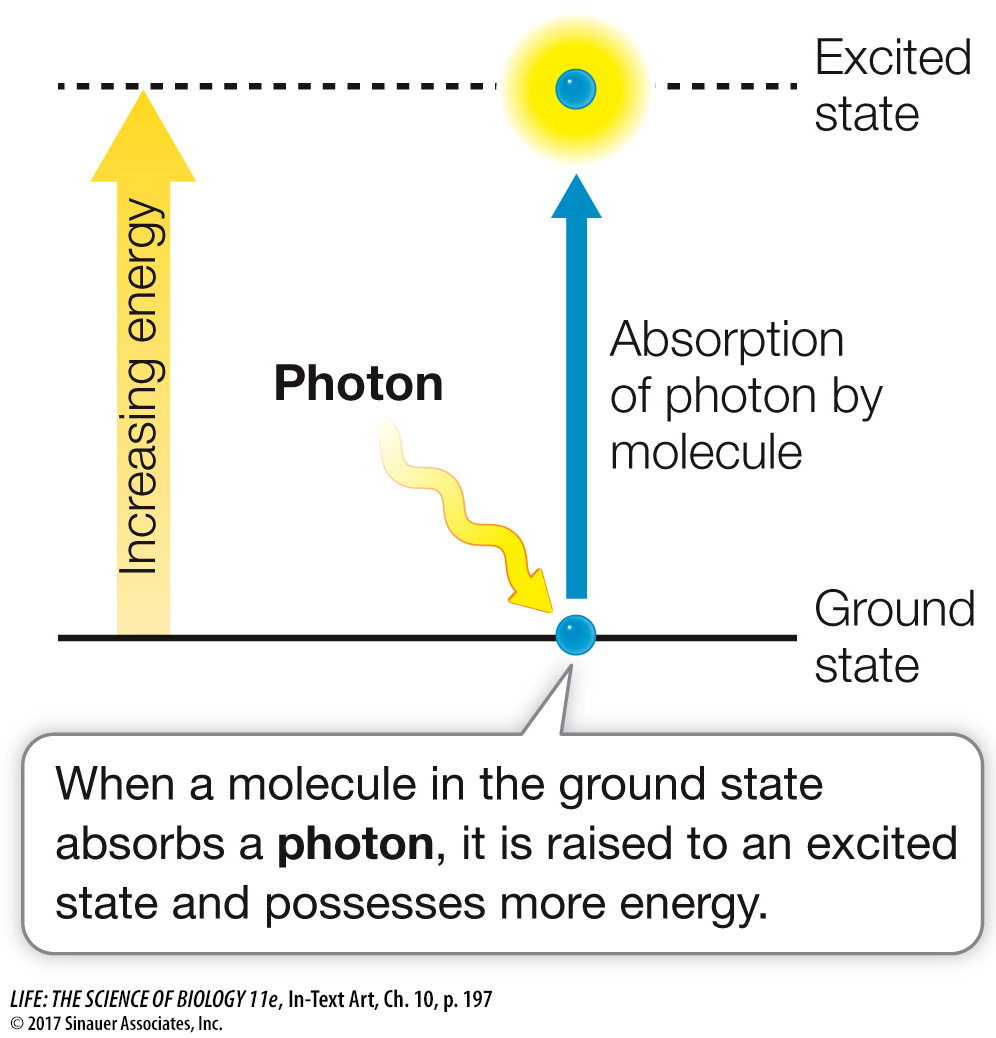
The difference in free energy between the molecule’s excited state and its ground state is approximately equal to the free energy of the absorbed photon (a small amount of energy is lost to entropy, according to the second law of thermodynamics). The increase in energy boosts one of the electrons in the molecule into a *shell farther from its nucleus; this electron is now held less firmly, making the molecule unstable and more chemically reactive.
*connect the concepts As discussed in Key Concept 2.1, the electrons orbiting an atom are distributed in a series of electron shells, or energy levels, around the nucleus. The farther an electron is from the nucleus, the higher its energy level.
PHOTOBIOLOGY The specific wavelengths absorbed by a particular molecule are characteristic of that type of molecule. Molecules that absorb wavelengths in the visible spectrum are called pigments.
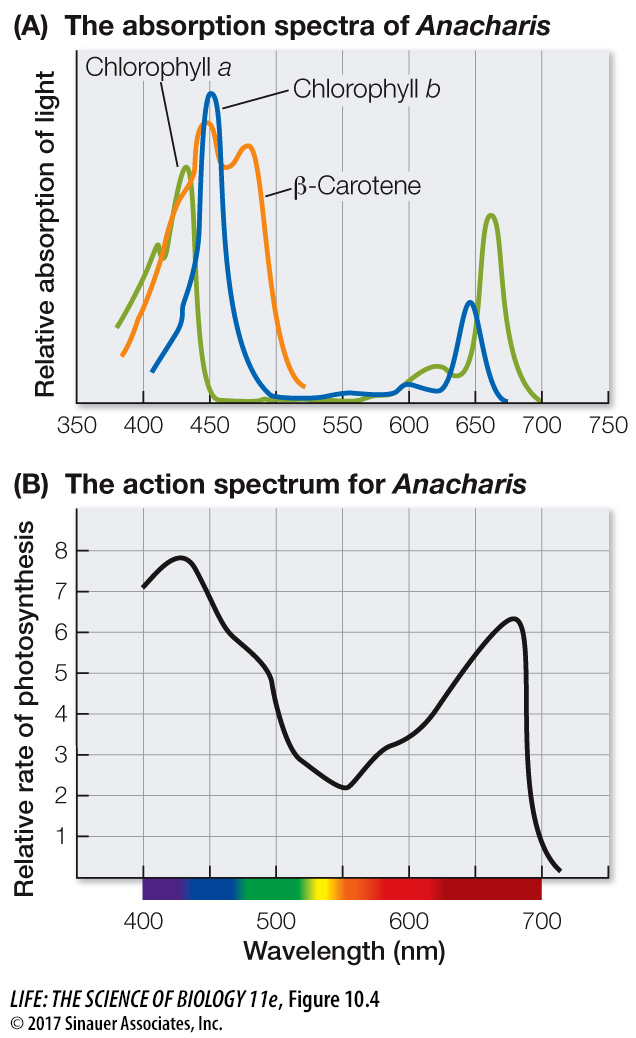
When a beam of white light (containing all the wavelengths of visible light) falls on a pigment, certain wavelengths are absorbed. The remaining wavelengths are scattered or transmitted and make the pigment appear to us as colored. For example, the pigment chlorophyll absorbs both blue and red light, and we see the remaining light, which is primarily green. If we plot light absorbed by a purified pigment against wavelength, the result is an absorption spectrum for that pigment.
In contrast to the absorption spectrum, an action spectrum is a plot of the rate of photosynthesis carried out by an organism against the wavelengths of light to which it is exposed. An action spectrum for photosynthesis can be determined as follows:
Place the organism in a closed container.
Expose it to light of a certain wavelength for a period of time.
Measure the rate of photosynthesis by the amount of O2 released.
Repeat with light of other wavelengths.
Figure 10.4 shows the absorption spectra of pigments in Anacharis, a common aquarium plant, and the action spectrum for photosynthesis by that plant. The two spectra can be compared to see which pigments in Anacharis contribute most to light harvesting for photosynthesis.
Q: Phycobilins are pigments that absorb yellow light. These pigments can transfer their absorbed energy to chlorophyll. How does this occur, thermodynamically?
In phycobilins, yellow light absorbs at a shorter wavelength (540 nm) that is more energetic than the longer wavelength (660 nm) at which chlorophyll absorbs. This means that the energy transfer from phycobilins to chlorophyll is thermodynamically favored (higher to lower energy).
The major pigment used to drive the light reactions of oxygenic photosynthesis is chlorophyll a. (In Figure 10.4 you can see that the wavelengths at which photosynthesis is highest in Anacharis are the same wavelengths at which chlorophyll a absorbs the most light.) Chlorophyll a has a complex ring structure (Figure 10.5), similar to that of the heme group of hemoglobin, with a magnesium ion at the center. A long hydrocarbon “tail” anchors the molecule to proteins within a large multi-
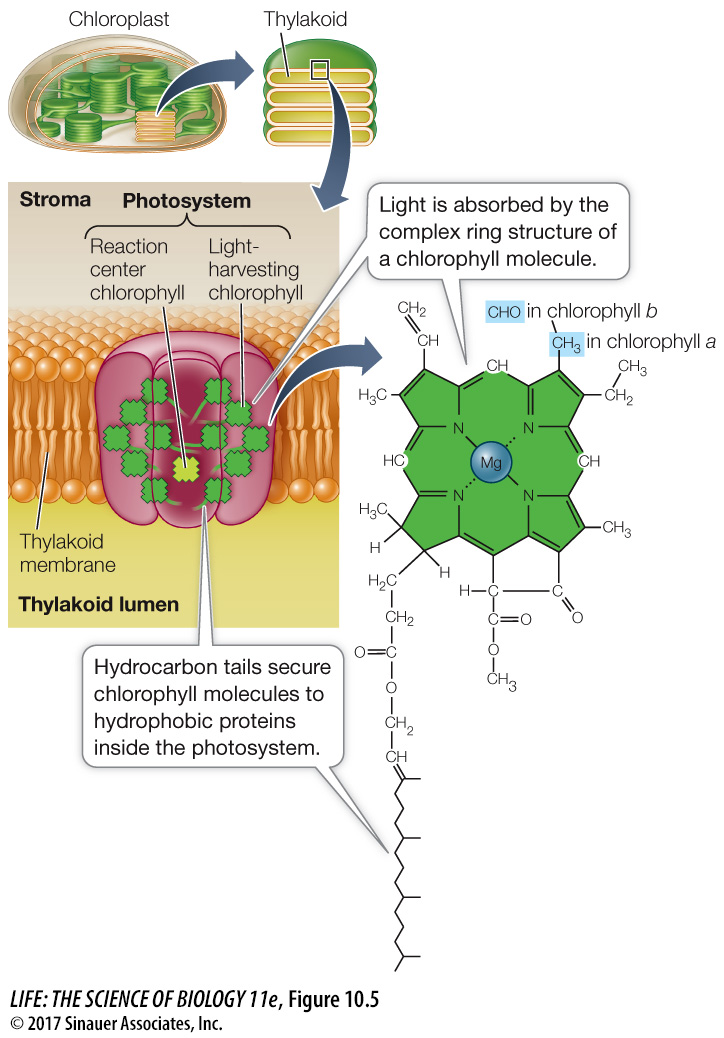
Light energy is captured by the light-
Chlorophyll absorbs blue and red light, which are near the two ends of the visible spectrum (see Figure 10.3). The various accessory pigments absorb light in other parts of the spectrum and thus function to broaden the range of wavelengths that can be used for photosynthesis. You can see this in the action spectrum in Figure 10.4B: Anacharis is able to photosynthesize at wavelengths of light that chlorophyll a doesn’t absorb but that other pigments absorb (e.g., 500 nanometers [nm]). Different photosynthetic organisms have different combinations of accessory pigments. Higher plants and green algae have chlorophyll b (with a structure and absorption spectrum very similar to that of chlorophyll a) and carotenoids such as β-carotene, which absorb photons in the blue and blue-