How did Watson and Crick deduce the structure of DNA?
Once pure DNA fibers could be isolated, biophysicists and biochemists examined the DNA for hints about its structure. The evidence eventually used to solve DNA’s structure included crucial data obtained using X-
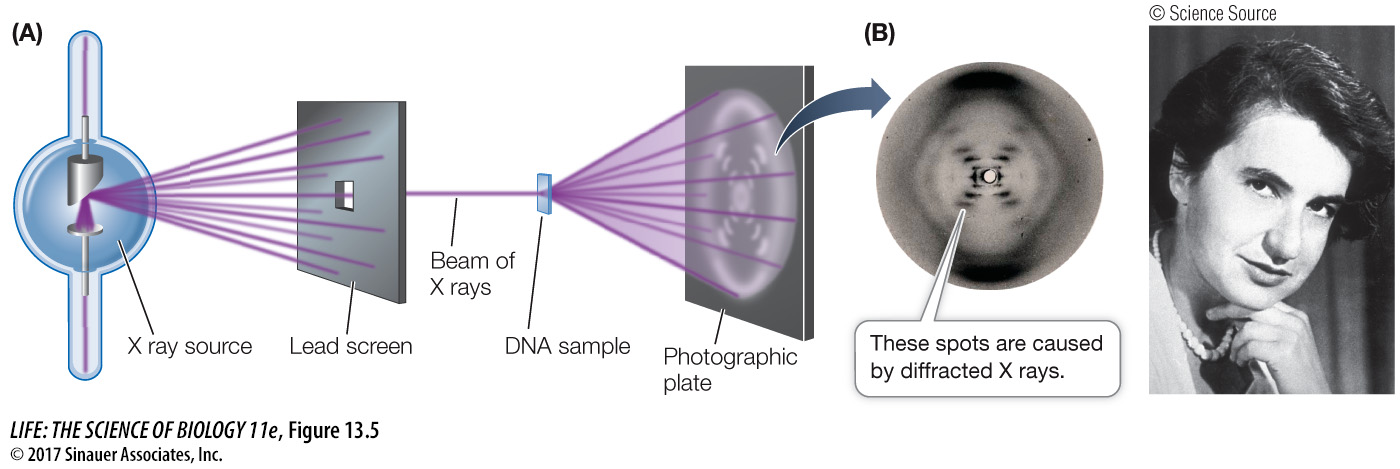
PHYSICAL EVIDENCE FROM X-
CHEMICAL EVIDENCE FROM BASE COMPOSITION Biochemists knew that DNA was a polymer of nucleotides. Each nucleotide consists of a molecule of the sugar deoxyribose, a phosphate group, and a nitrogen-
In the early 1950s, biochemist Erwin Chargaff and his colleagues at Columbia University reported that DNA from many different species—
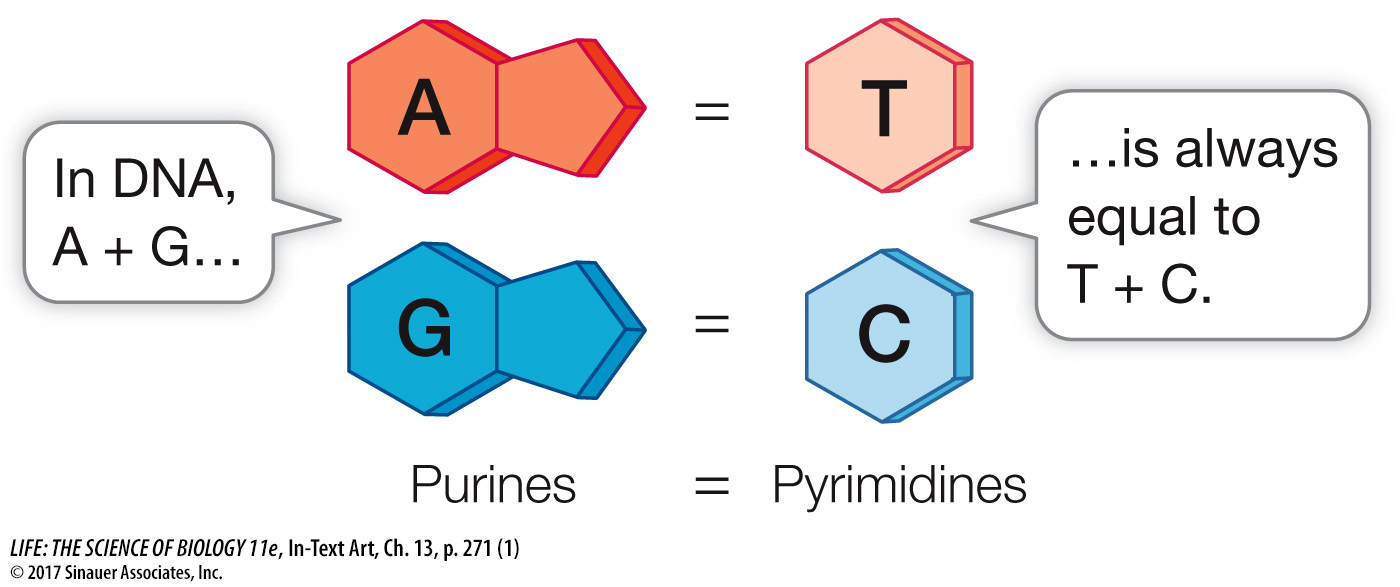
Chargaff’s rule provided an important clue about the way the bases are arranged in a DNA double helix. While Chargaff and colleagues found that this rule held for every organism they examined, they noted that the relative abundances of A + T versus G + C vary among organisms. In human DNA, A and T each account for 30 percent of the nitrogenous bases present, while G and C each account for 20 percent. Put another way, in human DNA there is a ratio of 60 percent (A + T) to 40 percent (C + G).
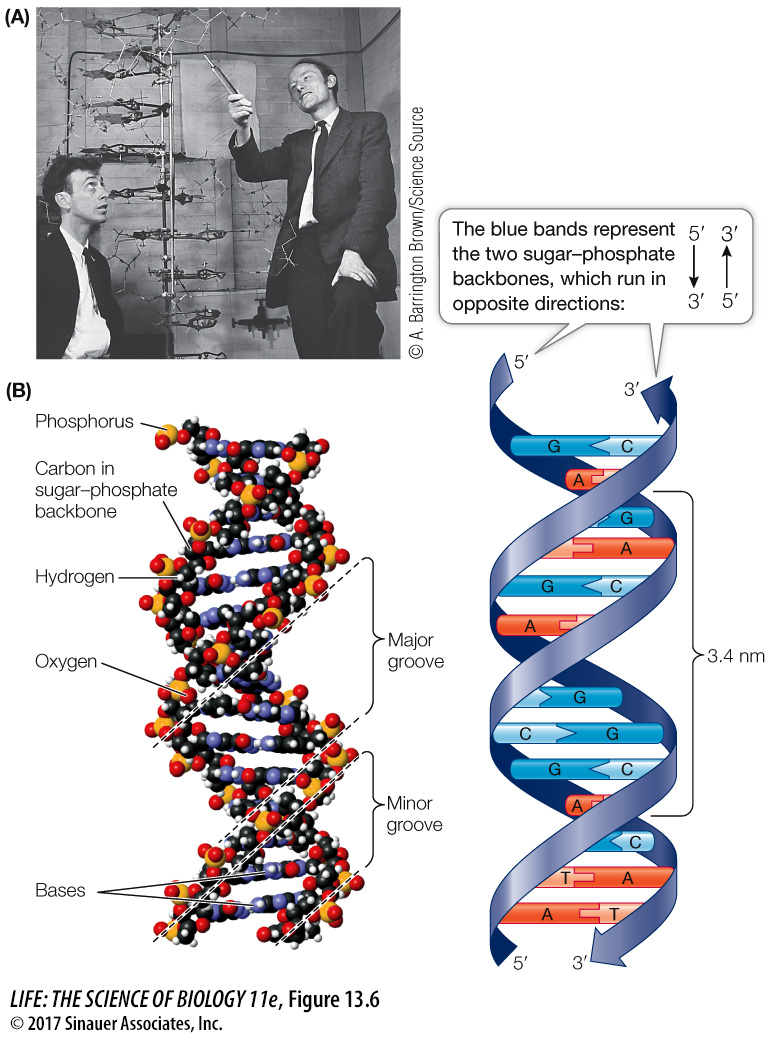
WATSON AND CRICK’S MODEL If you have taken chemistry courses, you may be familiar with model building, where balls (atoms) and sticks (bonds) are used to put together molecules based on known physical and chemical properties and bond angles. A physicist, Francis Crick, and a geneticist, James D. Watson (Figure 13.6A), who were then at the Cavendish Laboratory of Cambridge University, used model building to solve the structure of DNA. They used the physical and chemical evidence we just described:
To be consistent with Franklin’s X-
ray diffraction images, Watson and Crick’s model had the nucleotide bases on the interior of the two strands, with a sugar– phosphate “backbone” on the outside. In addition, the two DNA strands ran in opposite directions, that is, they were antiparallel. The two strands would not fit together otherwise: 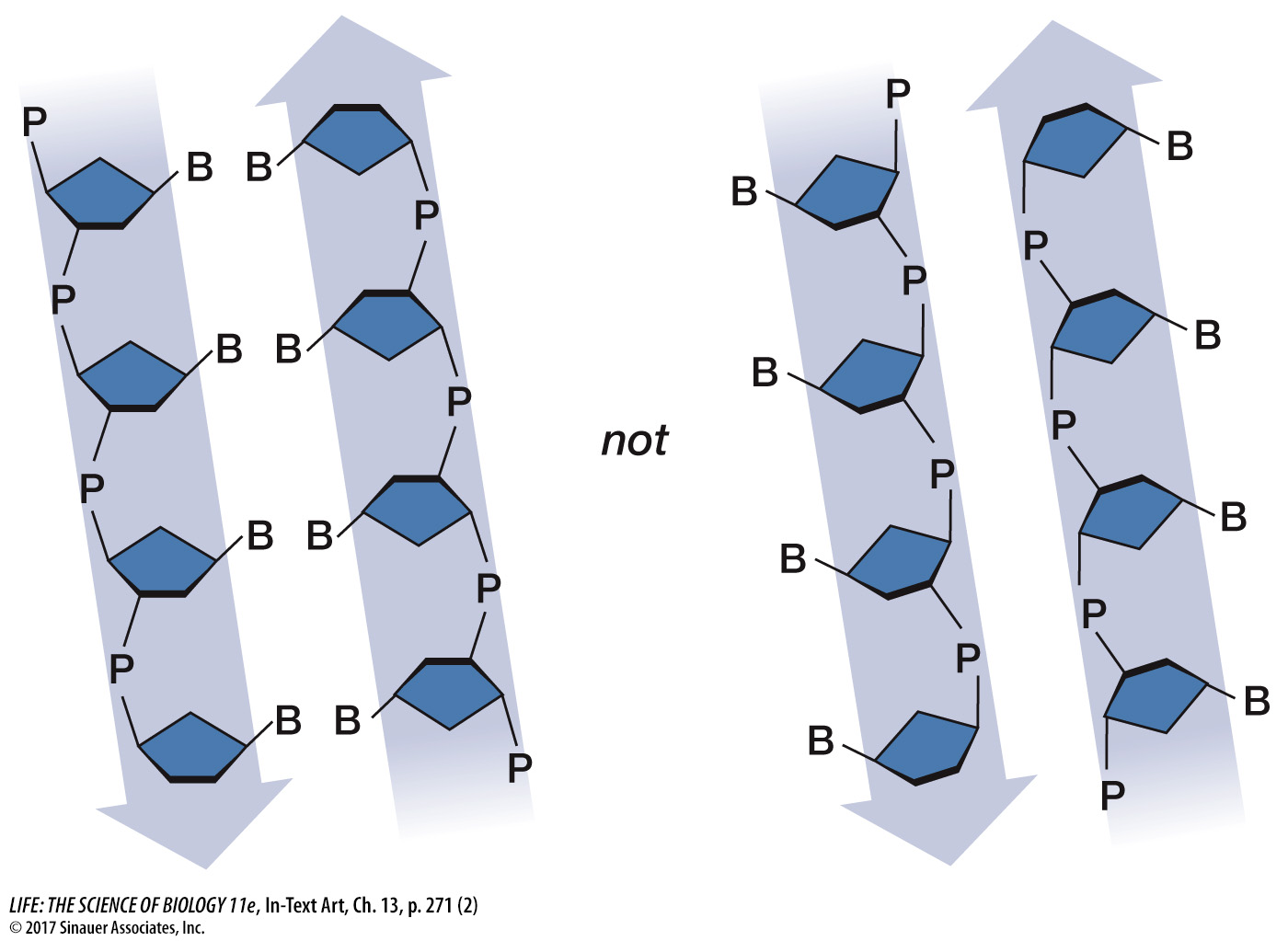
To satisfy Chargaff’s rule (A = T and G = C), Watson and Crick’s model always paired a purine on one strand with a pyrimidine on the opposite strand. These base pairs (A-
T and G- C) have the same width down the double helix, a uniformity that was also confirmed by X- ray diffraction: 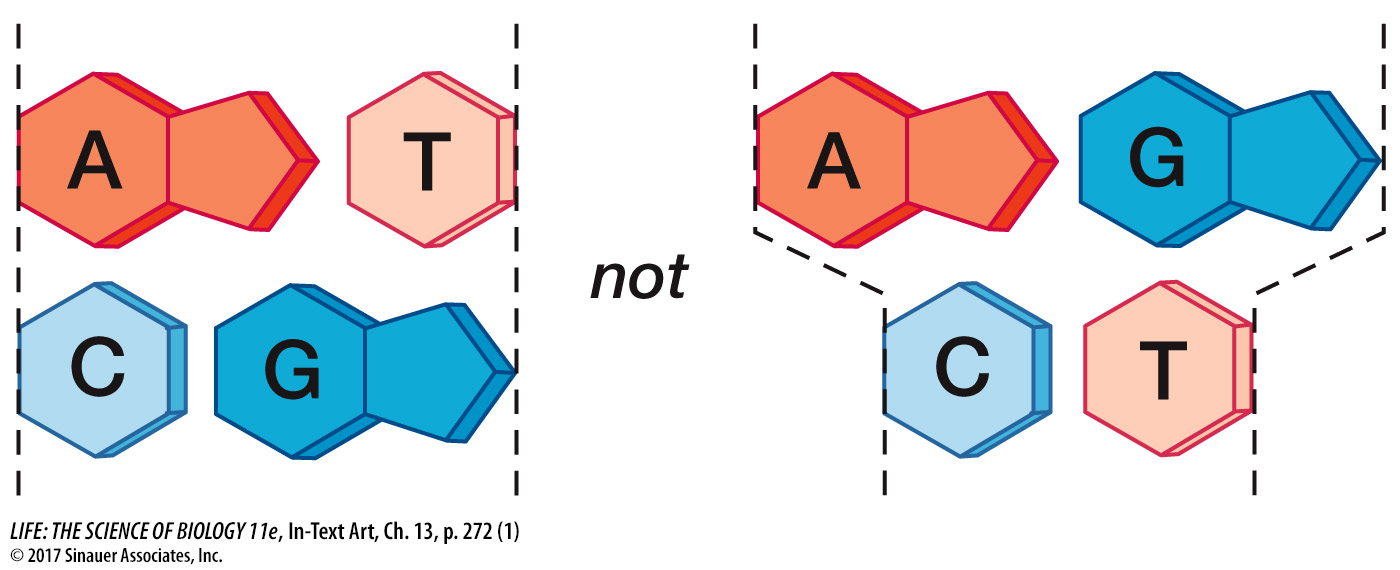
Crick and Watson built their tin model of DNA in late February 1953. This structure explained the known chemical properties of DNA, and it opened the door to understanding its biological functions.
Q: In DNA, where do the following chemical forces occur: hydrogen bonds, covalent bonds, and van der Waals forces?
Hydrogen bonds occur between the bases on opposite strands, within base pairs. Covalent bonds occur between the atoms that make up nucleotides and between the nucleotides in a DNA strand. van der Waals forces occur between the flat bases that stack on top of each other within the double helix, stabilizing them in the stacking.
Media Clip 13.1 Discovery of the Double Helix