The ribosome is the workbench for translation
The ribosome is the molecular workbench where the task of translation is accomplished. Its structure enables it to hold mRNA and charged tRNAs in the correct positions, thus allowing a polypeptide chain to be assembled efficiently. A given ribosome does not specifically produce just one kind of protein. A ribosome can use any mRNA and all species of charged tRNAs, and thus can be used to make many different polypeptide products. Ribosomes can be used over and over again, and there are thousands of them in a typical cell.
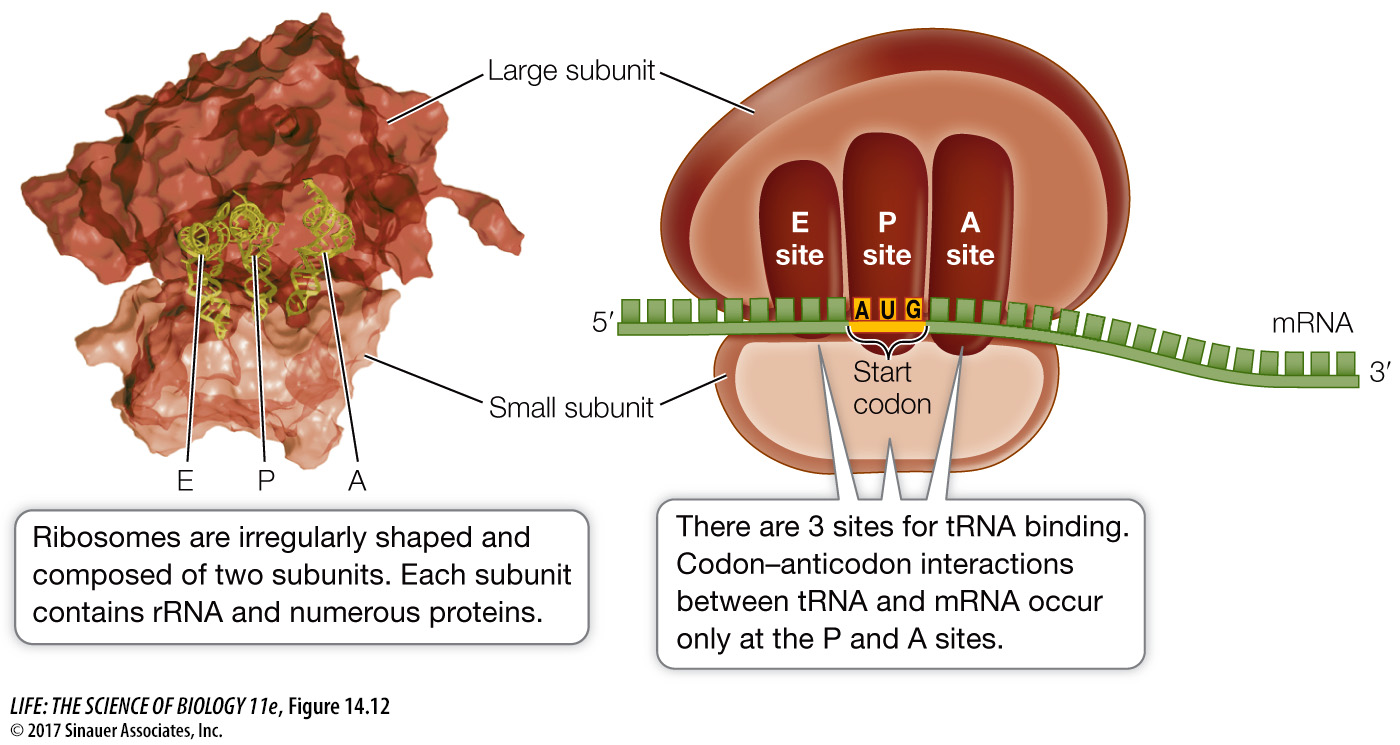
Although ribosomes are small relative to other cellular structures, their mass of several million daltons makes them large in comparison with charged tRNAs. Each ribosome consists of two subunits, a large one and a small one (Figure 14.12). The two subunits and several dozen other molecules interact noncovalently. In fact, when hydrophobic interactions between the proteins and RNAs are disrupted, the ribosome falls apart. The two subunits separate and all the RNAs and proteins separate from one another. If the disrupting agent is removed, the complex structure self-
Q: The ribosome consists of several dozen proteins and several RNA molecules, held together noncovalently. What are the chemical forces involved? How can these forces be disrupted and the molecules separated from one another?
The chemical forces that hold the molecules of the ribosome together include hydrogen bonds, ionic attractions, and hydrophobic interactions. These can be disrupted by heat or detergent.
In eukaryotes, the large subunit consists of three different molecules of ribosomal RNA (rRNA) and 49 different protein molecules, arranged in a precise configuration. The small subunit consists of 1 rRNA molecule and 33 different protein molecules. The ribosomes of prokaryotes are somewhat smaller than those of eukaryotes, and their ribosomal proteins and RNAs are different. Mitochondria and chloroplasts also contain ribosomes, some of which are similar to those of prokaryotes (see Chapter 5).
There are three sites to which a tRNA can bind on the large subunit of the ribosome, designated A, P, and E (see Figure 14.12). The mRNA and ribosome move in relation to one another, and as they do so, a charged tRNA traverses these three sites in order:
The A (aminoacyl-
tRNA) site is where the charged tRNA anticodon binds to the mRNA codon, thus lining up the correct amino acid to be added to the growing polypeptide chain.The P (peptidyl-
tRNA) site is where the tRNA adds its amino acid to the polypeptide chain.The E (exit) site is where the tRNA, having given up its amino acid, resides before being released from the ribosome and going back to the cytosol to pick up another amino acid and begin the process again.
The ribosome has a fidelity function that ensures that the mRNA–