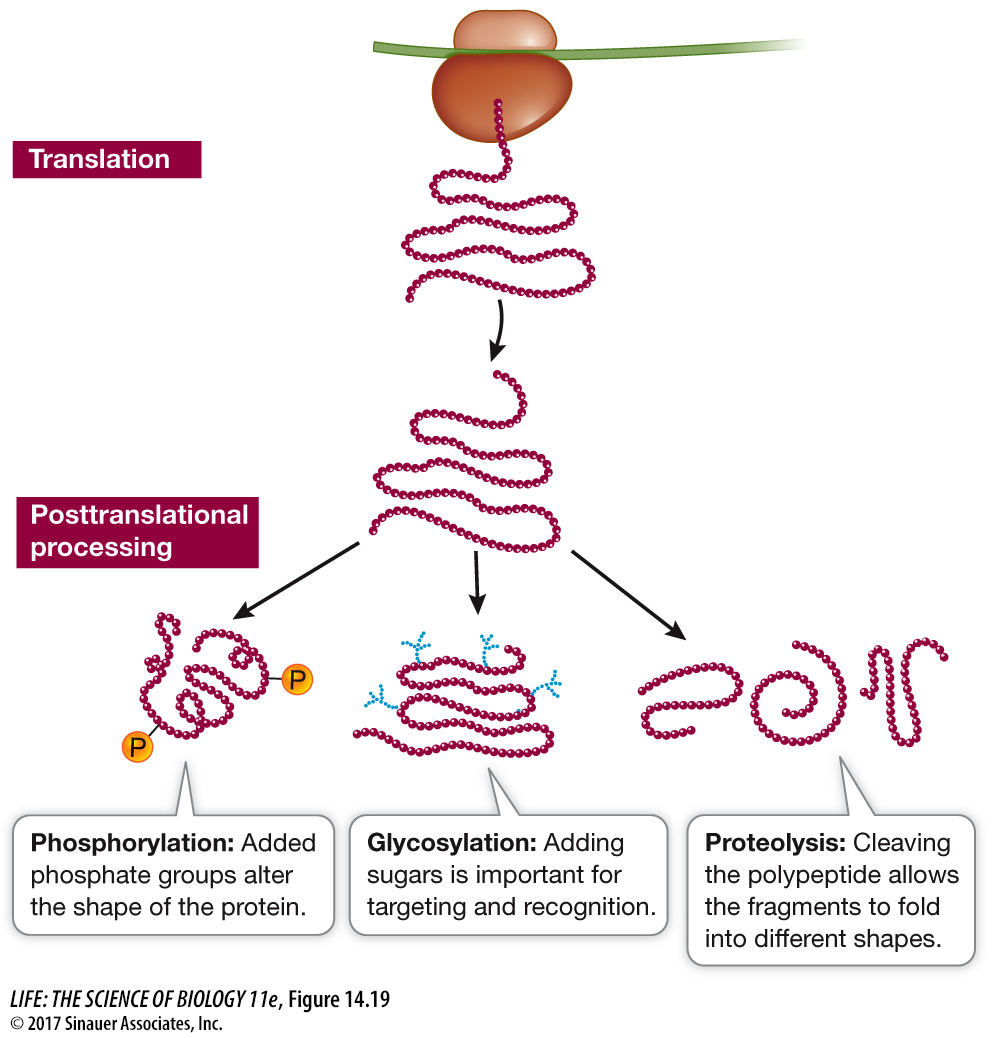
Many proteins are modified after translation
The amino acid sequences of most mature proteins are not identical to those in the polypeptide chains that are translated from mRNA on the ribosomes, because polypeptides are often modified in any of several ways after translation (Figure 14.19). These modifications are essential to the final functioning of the protein:
Proteolysis is the cutting of a polypeptide chain, a reaction catalyzed by enzymes called proteases (also called peptidases or proteinases). Cleavage of the signal sequence from the growing polypeptide chain in the RER is an example of proteolysis (see Figure 14.17); the protein might move back out of the RER through the membrane channel if the signal sequence were not cut off. Some proteins are actually made from polyproteins (long polypeptides) that are cut into final products by proteases. These proteases are essential to some viruses, including human immunodeficiency viruses (HIVs), because the large viral polyprotein cannot fold properly unless it is cut. Certain drugs used to treat acquired immune deficiency syndrome (AIDS) work by inhibiting the HIV protease, thereby preventing the formation of proteins needed for viral reproduction.
Glycosylation is the addition of sugars to proteins to form glycoproteins. In both the RER and the Golgi apparatus, resident enzymes catalyze the addition of various sugars or short sugar chains to certain amino acid R groups on proteins. One such type of “sugar coating” is essential for directing proteins to lysosomes, as mentioned above. Other types are important in the conformation of proteins and their recognition functions at the cell surface (see Key Concept 6.2). Other attached sugars help stabilize extracellular proteins, or proteins stored in vacuoles in plant seeds.
Phosphorylation is the addition of phosphate groups to proteins, and is catalyzed by protein kinases. The charged phosphate groups change the conformation of a protein, often exposing the active site of an enzyme or the binding site for another protein. We have seen the important role of phosphorylation in cell signaling (see Chapter 7) and the cell cycle (see Chapter 11).