Translation of mRNA can be regulated by proteins
From what we have described in this chapter so far, you may get the impression that in eukaryotes all regulation of gene expression is at the level of transcription. But is the amount of a protein in a cell really determined only by the amount of its mRNA? The answer is no. For example, a survey of genes and their expression in yeast cells showed that for about one-
They can regulate translation of the protein’s mRNA.
They can regulate how long a newly synthesized protein persists in the cell (protein longevity).
REGULATION OF TRANSLATION There are a variety of ways in which the translation of mRNA can be regulated. One way, as we saw in the previous section, is to inhibit translation with siRNAs and miRNAs. A second way involves modification of the guanosine triphosphate cap on the 5′ end of the mRNA (see Key Concept 14.4). An mRNA that is capped with an unmodified GTP molecule is not translated. For example, stored mRNAs in the egg cells of the tobacco hornworm moth are capped with unmodified GTP molecules and are not translated. After the egg is fertilized, however, the caps are modified, allowing the mRNA to be translated to produce the proteins needed for early embryonic development.
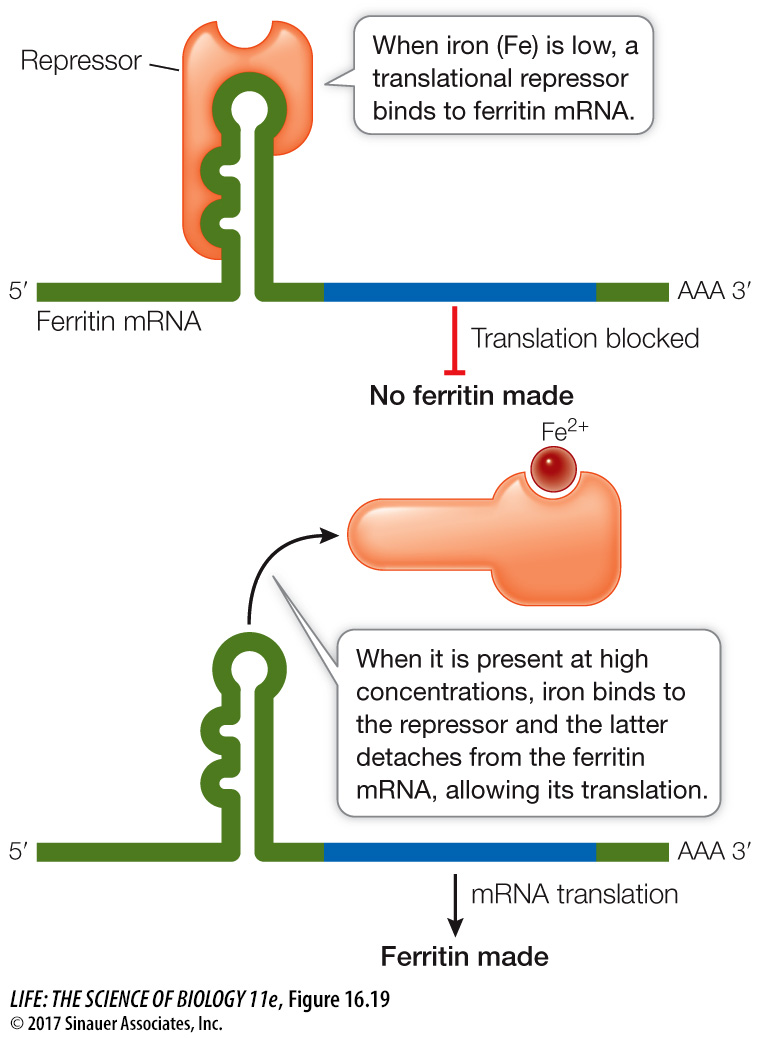
In another system, repressor proteins directly block translation. For example, in mammalian cells the protein ferritin binds free iron ions (Fe2+). When iron is present in excess, ferritin synthesis rises dramatically, but the amount of ferritin mRNA remains constant, indicating that the increase in ferritin synthesis is due to an increased rate of mRNA translation. Indeed, when the iron level in the cell is low, a translational repressor protein binds to the 5′ noncoding region of ferritin mRNA and prevents its translation by blocking its attachment to a ribosome. When the iron level rises, some of the excess Fe2+ ions bind to the repressor and alter its three-
REGULATION OF PROTEIN LONGEVITY The protein content of a cell at any given time is a function of both protein synthesis and protein degradation. Certain proteins can be targeted for destruction in a chain of events that begins when an enzyme attaches a 76-
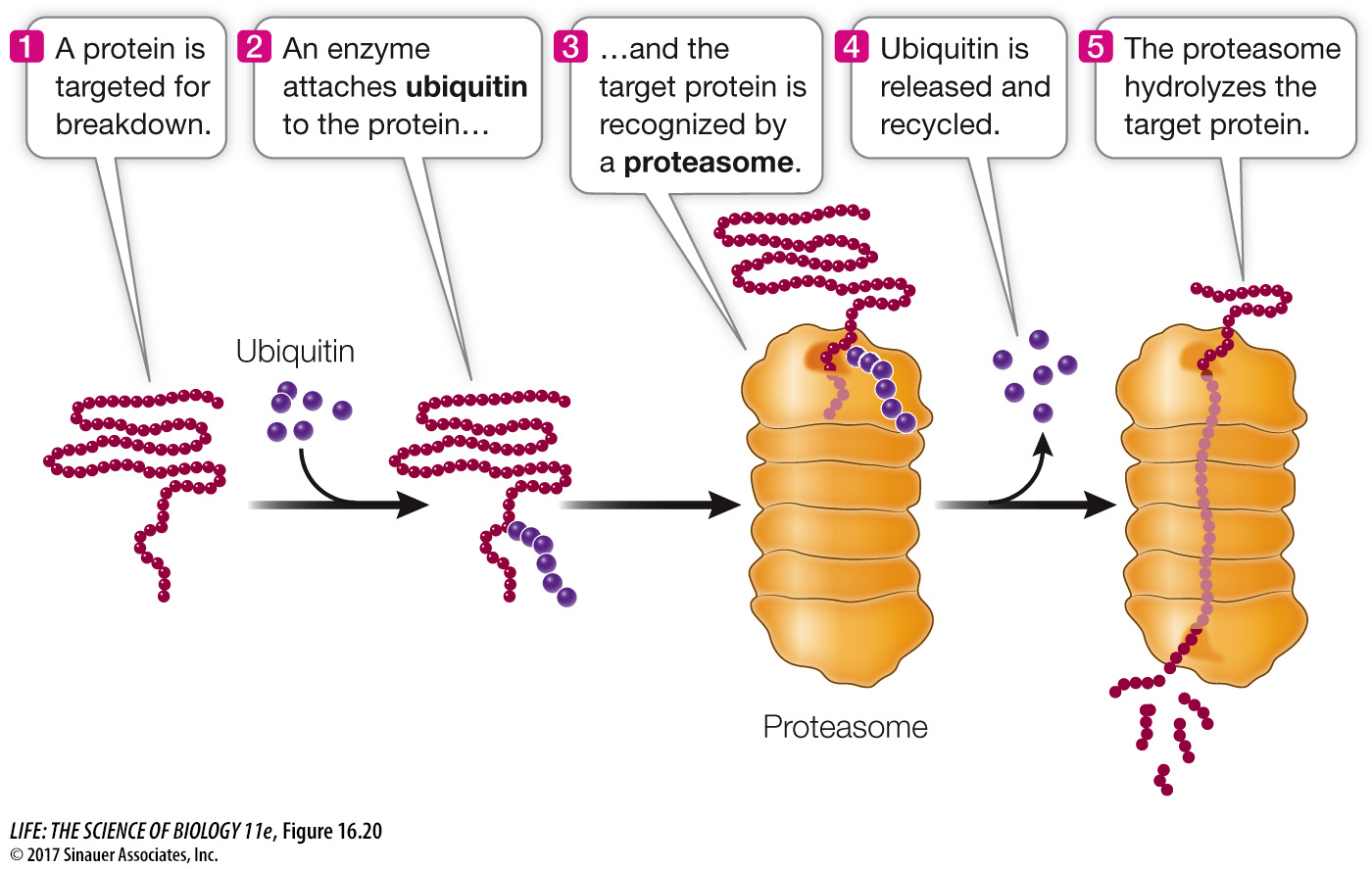
You may recall from Key Concept 11.2 that cyclins are proteins that regulate the activities of key enzymes at specific points in the cell cycle. Cyclins must be broken down at just the right time, and this is done by attaching ubiquitin to them and degrading them in the proteasomes. Viruses can hijack this system. For example, some strains of the human papillomavirus (HPV) add ubiquitin to the p53 and retinoblastoma proteins, targeting them for proteasomal degradation. These proteins normally inhibit the cell cycle, so the result of this HPV activity is unregulated cell division (cancer).