Inserted DNA is usually integrated into the host chromosome
Cells can be chemically treated to make their outer membranes more permeable, and then mixed with the DNA so that it can diffuse into the cells. Another approach is called electroporation: a short electric shock is used to create temporary pores in the membranes through which the DNA can enter. Viruses can be altered so that they carry recombinant DNA into cells. A common method for transforming plants involves a specific bacterium that inserts DNA into plant cells. Transgenic animals can be produced by injecting recombinant DNA into the nuclei of fertilized eggs. There are even “gene guns” that “shoot” the host cells with tiny metal particles coated with the DNA.
The challenge of inserting new DNA into a cell lies not only in getting it into the host cell, but also in getting it to replicate as the host cell divides. As you saw in Key Concept 13.3, DNA polymerase does not bind to and copy just any sequence. If the new DNA is to be replicated, it must become part of a segment of DNA that contains an origin of replication. Such a DNA molecule is called a replicon, or replication unit.
There are two general ways in which the newly introduced DNA can become part of a replicon within the host cell:
It can be spliced into a host chromosome directly.
It can enter the host cell as part of a carrier DNA sequence, called a vector, and can then either integrate into a host chromosome or be replicated from its own origin of replication.
Several types of vectors are used to get DNA into cells, some of which we will now describe in more detail.
PLASMIDS AS VECTORS As we described in Chapter 12, plasmids are small, circular DNA molecules that replicate autonomously in many prokaryotic cells. Several characteristics make plasmids useful as transformation vectors:
Plasmids are relatively small (an E. coli plasmid usually has 2,000–
6,000 base pairs) and are therefore easy to manipulate in the laboratory. Page 384A plasmid often has one or more restriction enzyme recognition sequences that each occur only once in the plasmid sequence. This makes it easy to insert new DNA into the prokaryotic plasmid before it is used to transform host cells.
Many plasmids contain genes that confer resistance to antibiotics, which can serve as selectable markers.
Plasmids have a bacterial origin of replication (ori) and can replicate independently of the host chromosome. It is not uncommon for a bacterial cell to contain hundreds of copies of a recombinant plasmid. For this reason, the power of bacterial transformation to amplify a gene is extraordinary. A 1-
liter culture of bacteria harboring the human β-globin gene in a typical plasmid has as many copies of that gene as there are cells in a typical adult human (1014).
A plasmid used as a vector in the laboratory has been extensively altered to include convenient features:
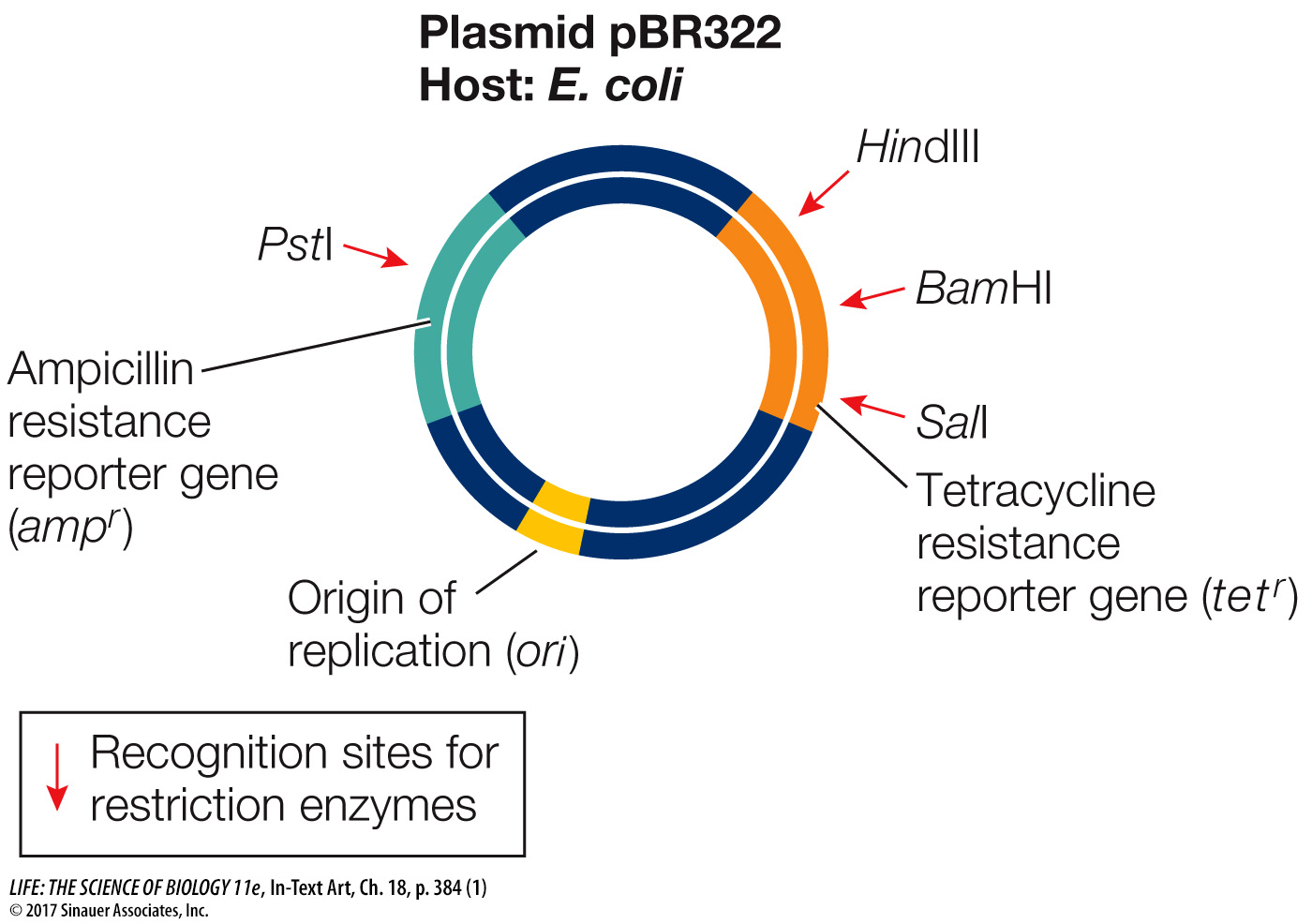
Multiple cloning sites, often with 20 or more unique restriction enzyme sites for cloning purposes (see HindIII, BamHI, SalI, and PstI in the diagram below)
An origin of replication that will function in a particular type of host cell (see ori in the diagram below)
One or more reporter genes, such as selectable marker genes (see ampicillin resistance and tetracycline resistance in the diagram below)
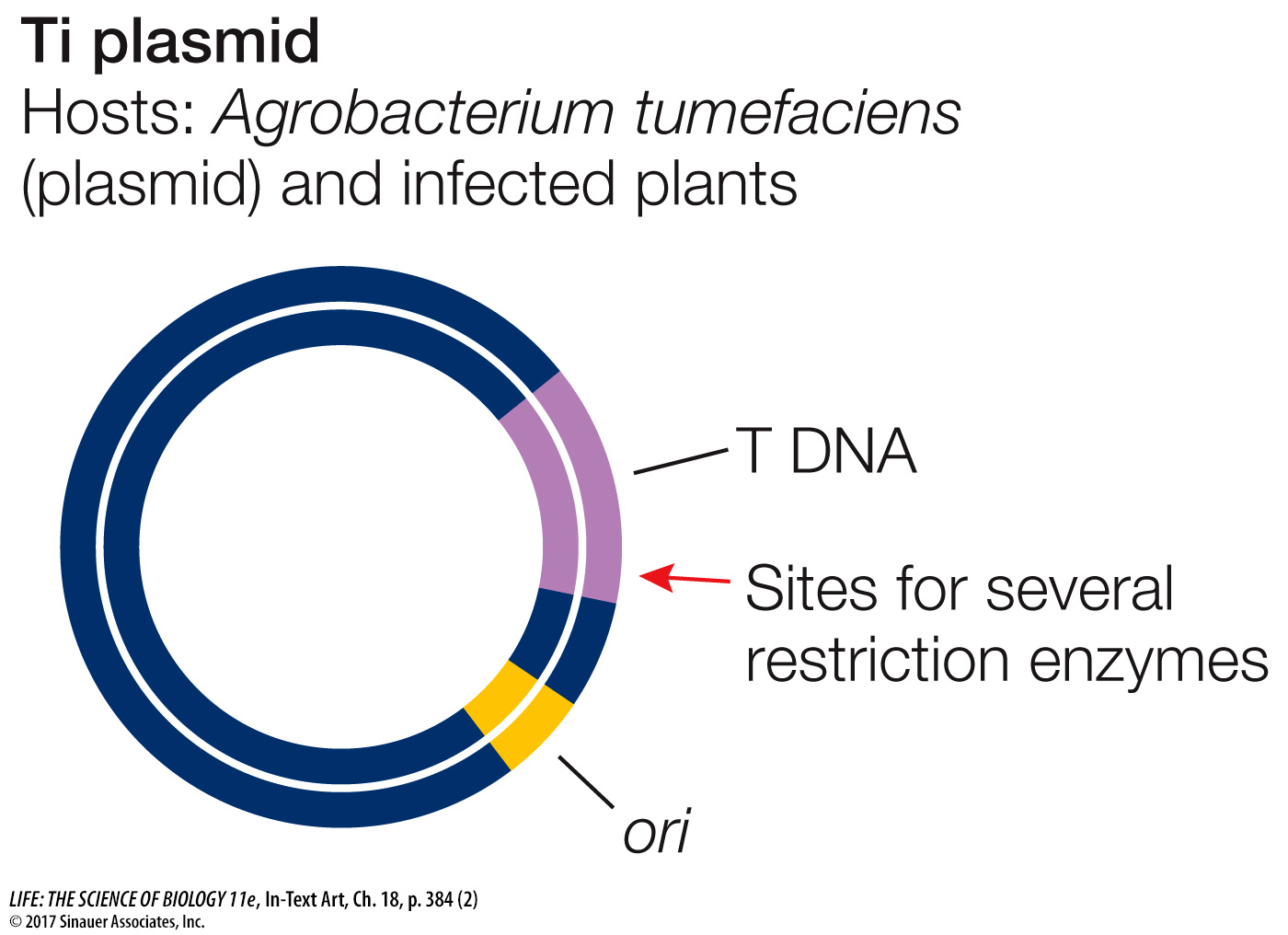
PLASMID VECTORS FOR PLANTS An important vector for carrying new DNA into many types of plants is a plasmid found in the bacterium Agrobacterium tumefaciens. This bacterium lives in the soil, infects plants, and causes a disease called crown gall, which is characterized by the presence of growths (or tumors) on the plant. Agrobacterium contains a plasmid called Ti (for tumor-
When used as a vector for plant transformation, the tumor-
VIRUSES AS VECTORS Constraints on plasmid replication limit the size of the new DNA that can be inserted into a plasmid to about 10,000 base pairs. Although many prokaryotic genes may be smaller than this, most eukaryotic genes—
Both prokaryotic and eukaryotic viruses are used as vectors for eukaryotic DNA. Bacteriophage λ, which infects E. coli, has a DNA genome of about 45,000 base pairs. About 20,000 base pairs are not necessary for the bacteriophage to complete its life cycle (see Figure 16.12). These 20,000 base pairs can be deleted and replaced with DNA from another organism, which then gets replicated along with the virus DNA. Because viruses infect cells naturally, they offer a great advantage over plasmids, which often require artificial means to coax them to enter host cells.