A cascade of transcription factors establishes body segmentation in the fruit fly
Perhaps the best-
The life cycle of Drosophila from fertilized egg to adult takes about 2 weeks at room temperature. The egg hatches into a larva, which then forms a pupa, which finally is transformed into the adult fly. By the time a larva appears—
As in other organisms, fertilization in Drosophila leads to a rapid series of mitoses. However, the first 12 cycles of nuclear division are not accompanied by cytokinesis. So a multinucleate embryo forms instead of a multicellular embryo (the nuclei are brightly stained in the micrographs below):
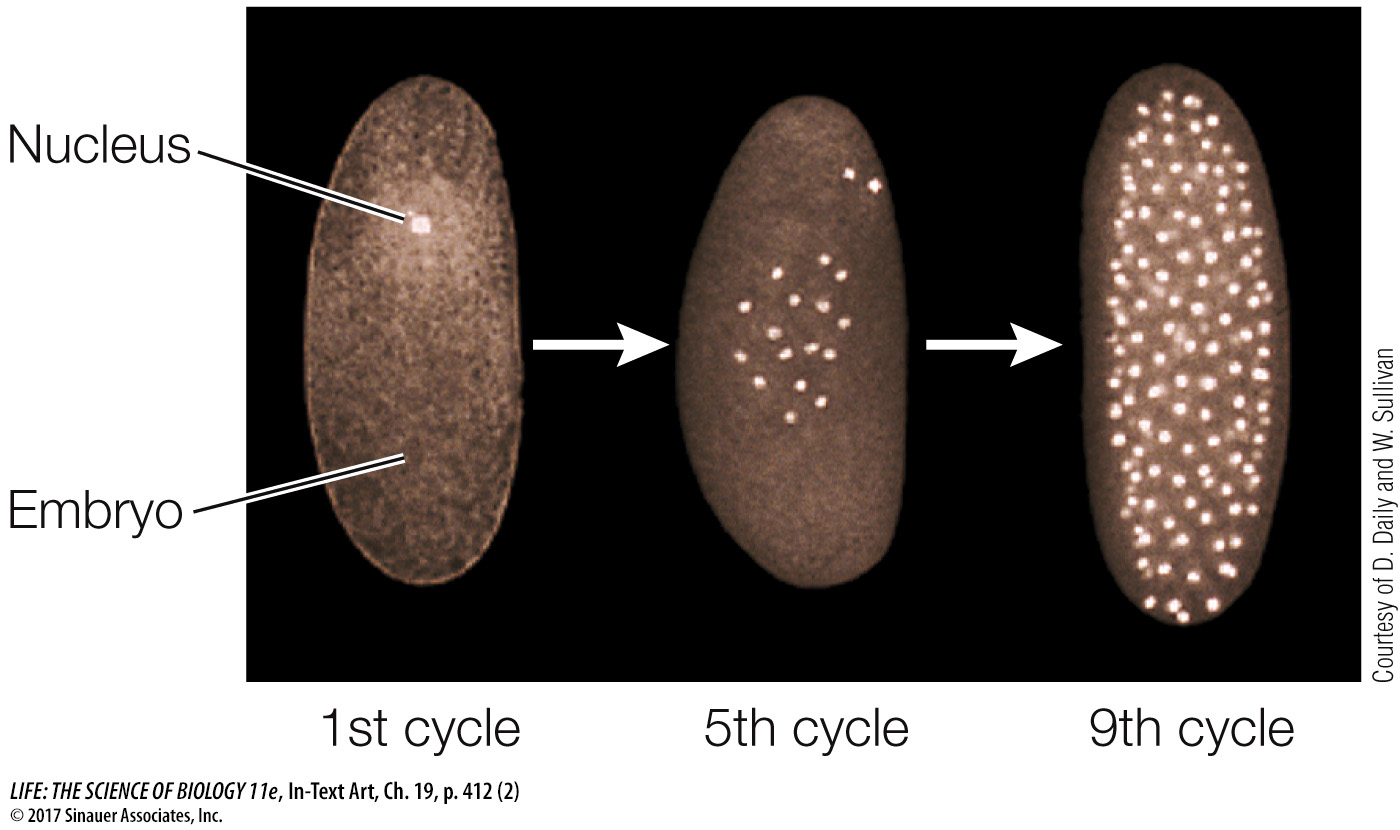
With no cell membranes to cross, morphogens can diffuse easily within the embryo. We focus here on the determination events that occur in the first 24 hours after fertilization. We’ll present what may appear to be a lot of detail but is really just an outline of the molecular events. In describing them, biologists are getting closer to answering a key question: How does a complex organism develop from a single cell?
Biologists used experimental genetics to describe the events leading to cell fate determination:
First, developmental mutations were identified. For example, a mutant strain might produce larvae with two heads or no segments.
Then the mutant flies were compared with wild-
type flies, and the gene responsible for the developmental mistake, and the gene’s protein product (if appropriate), were isolated. Finally, experiments with the gene (making transgenic flies) and protein (injecting the protein into an egg or into an embryo) were done to confirm their roles in the proposed developmental pathway.
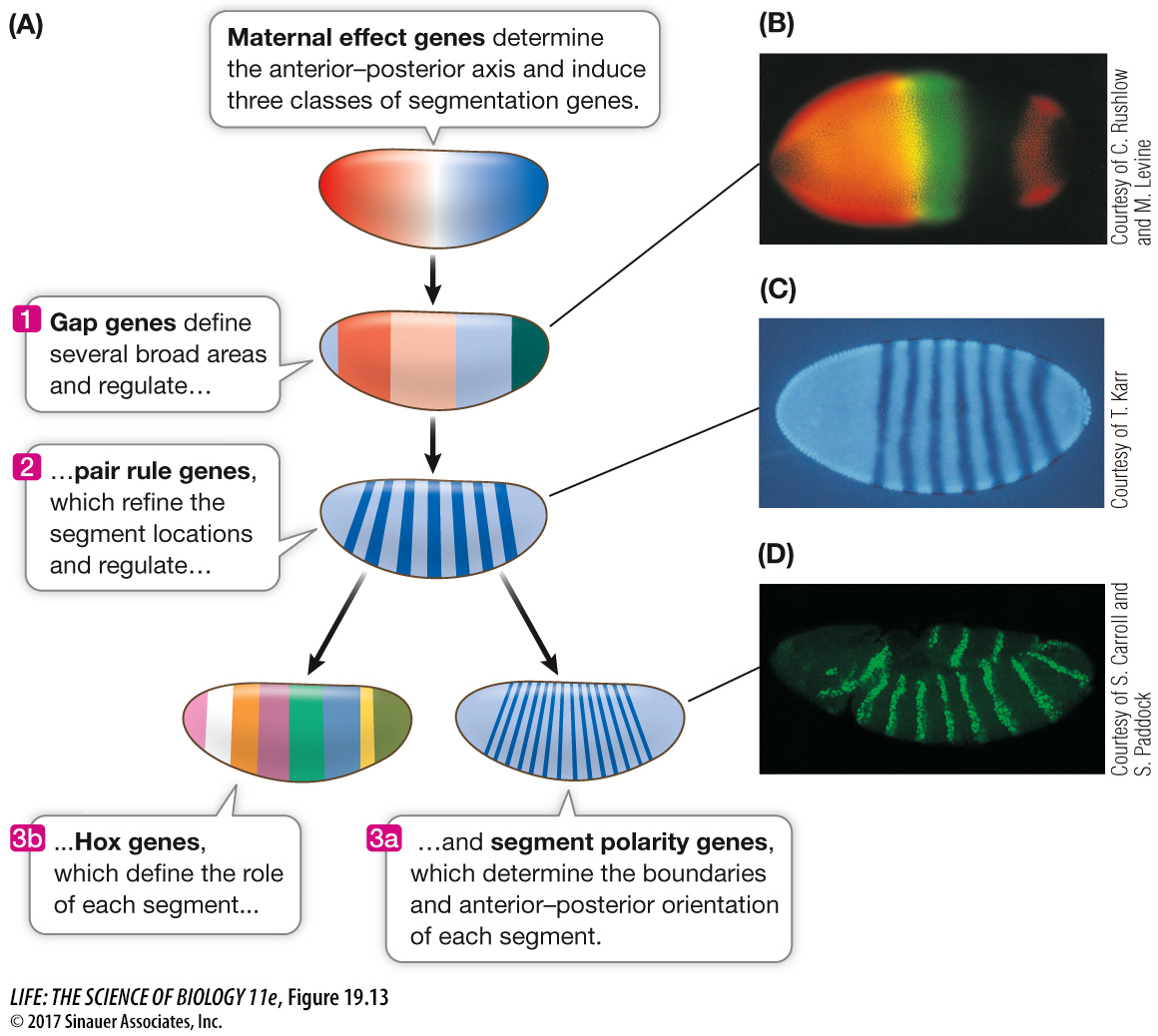
These approaches revealed an amazing cascade of gene expression events that result in the determination of each segment within 24 hours after fertilization. Several classes of genes are involved:
Maternal effect genes set up the major axes (anterior–
posterior and dorsal– ventral) of the egg. Segmentation genes determine the boundaries and polarity of each segment.
Hox genes determine which organ will be made at a given location.
MATERNAL EFFECT GENES Like the eggs and early embryos of sea urchins, Drosophila eggs and larvae are characterized by unevenly distributed cytoplasmic determinants (see Figure 19.6). These molecular determinants are the products of specific maternal effect genes that are transcribed in the cells of the mother’s ovary. Two maternal effect genes called bicoid and nanos help determine the anterior–
The mRNAs for bicoid and nanos diffuse from the mother’s cells and are passed to the egg by cytoplasmic bridges into what will be the anterior end of the egg. The bicoid mRNA is translated into Bicoid protein, a transcription factor that diffuses away from the anterior end, establishing a gradient in the egg cytoplasm (Figure 19.12A). Meanwhile, the egg’s cytoskeleton transports the nanos mRNA from the anterior end of the egg, where it was deposited, to the posterior end, where it is translated (Figure 19.12B).
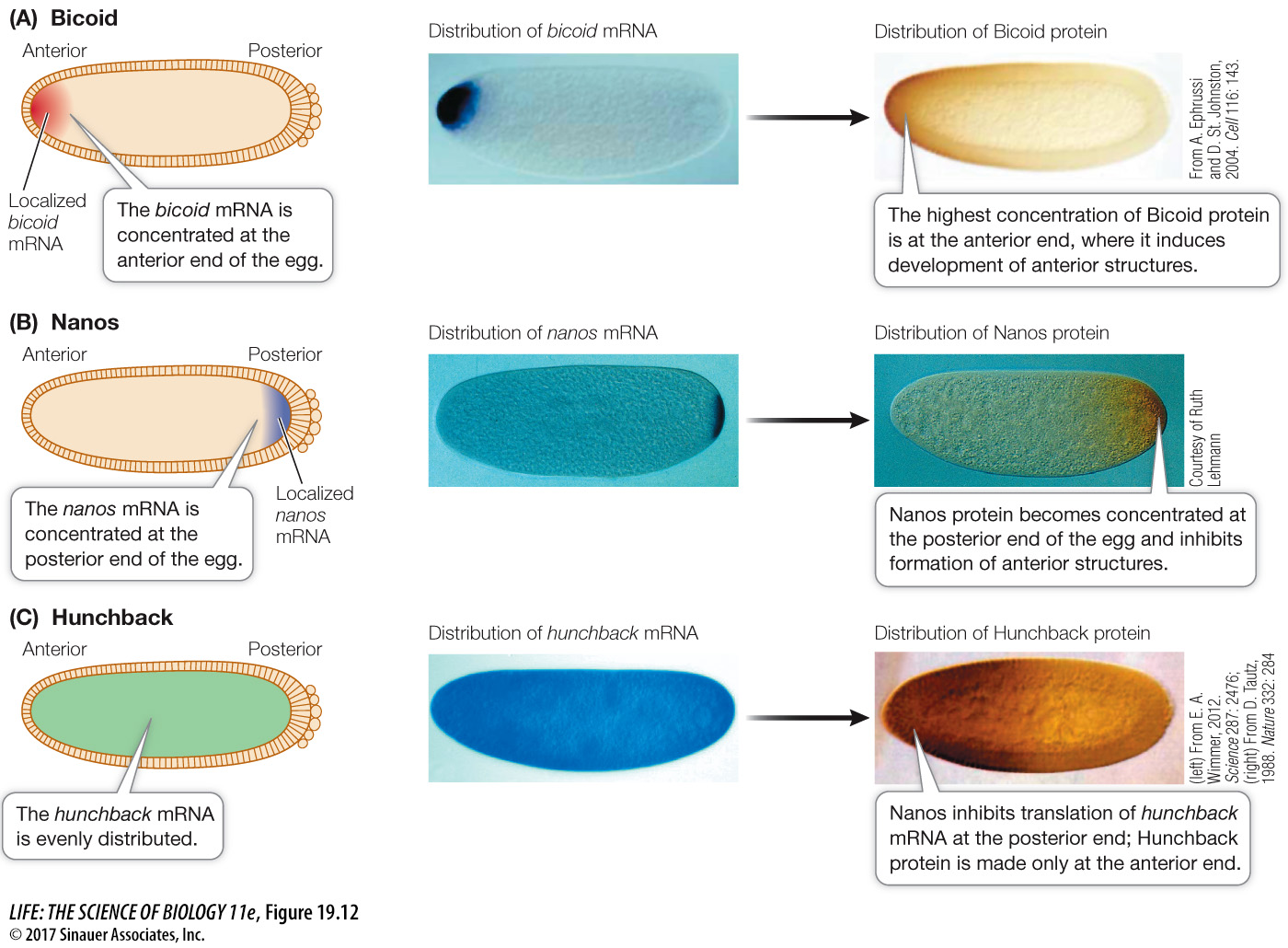
The actions of Bicoid and Nanos establish a gradient of yet another protein, called Hunchback, which determines the anterior and posterior ends of the embryo. Initially, the hunchback mRNA is evenly distributed in the embryo, but Nanos inhibits its translation, thus preventing Hunchback protein accumulation at the posterior end of the embryo (Figure 19.12C). Meanwhile, at the anterior end of the embryo, Bicoid stimulates increased transcription of the hunchback gene, thus increasing the amount of hunchback mRNA (and thus Hunchback protein) and further strengthening the Hunchback gradient.
How did biologists elucidate these pathways? Let’s look at the experimental approaches used in this case.
Females that are homozygous for a particular bicoid mutation produce larvae with no head and no thorax; thus the Bicoid protein must be needed for the anterior structures to develop.
If the eggs of these bicoid mutant flies are injected at the anterior end with cytoplasm from the anterior region of a wild-
type egg, the injected eggs develop into normal larvae. This experiment also shows that the Bicoid protein is involved in the development of anterior structures. If cytoplasm from the anterior region of a wild-
type egg is injected into the posterior region of another egg, anterior structures develop there. The degree of induction depends on how much cytoplasm is injected. Eggs from homozygous nanos mutant females develop into larvae with missing abdominal segments.
If cytoplasm from the posterior region of a wild-
type egg is injected into the posterior region of a nanos mutant egg, it will develop normally.
The events involving bicoid, nanos, and hunchback begin before fertilization and continue after it, during the multinucleate stage, which lasts a few hours. At this stage the embryo looks like a bunch of indistinguishable nuclei under the light microscope. But a lot is going on at the molecular level, as cell fates have already begun to be determined. After the anterior and posterior ends have been established, the next step in pattern formation is the determination of segment number and locations.
SEGMENTATION GENES These genes determine the number and polarity of the Drosophila larval segments and are expressed when there are about 6,000 nuclei in the embryo (about 3 hours after fertilization). Three classes of segmentation genes act one after the other to regulate finer and finer details of the segmentation pattern:
Gap genes organize broad areas along the anterior–
posterior axis. Mutations in gap genes result in gaps in the body plan— the omission of several consecutive larval segments. Pair rule genes divide the embryo into units of two segments each. Mutations in pair rule genes result in embryos missing every other segment.
Segment polarity genes determine the boundaries and anterior–
posterior organization of the individual segments. Mutations in segment polarity genes can result in segments in which posterior structures are replaced by reversed (mirror- image) anterior structures.
The expression of these genes is sequential (Figure 19.13). The products of the gap genes activate pair rule genes, and the pair rule gene products activate segment polarity genes. By the end of this cascade, nuclei throughout the embryo “know” which segment they will be part of in the adult fly.
Animation 19.3 Pattern Formation in the Drosophila Embryo
Media Clip 19.1 Spectacular Fly Development in 3D
The next set of genes in the cascade determines the form and function of each segment.
HOX GENES Hox (for “Homeobox”) genes encode a family of transcription factors that are expressed in different combinations along the length of the embryo, and help determine cell fate within each segment. Hox gene expression tells the cells of a segment in the head to make eyes, those of a segment in the thorax to make wings, and so on. The Drosophila Hox genes occur in two clusters on chromosome 3, in the same order as the segments whose function they determine (Figure 19.14). By the time the fruit fly larva hatches, its segments are completely determined. Hox genes are shared by all animals and are homeotic genes—
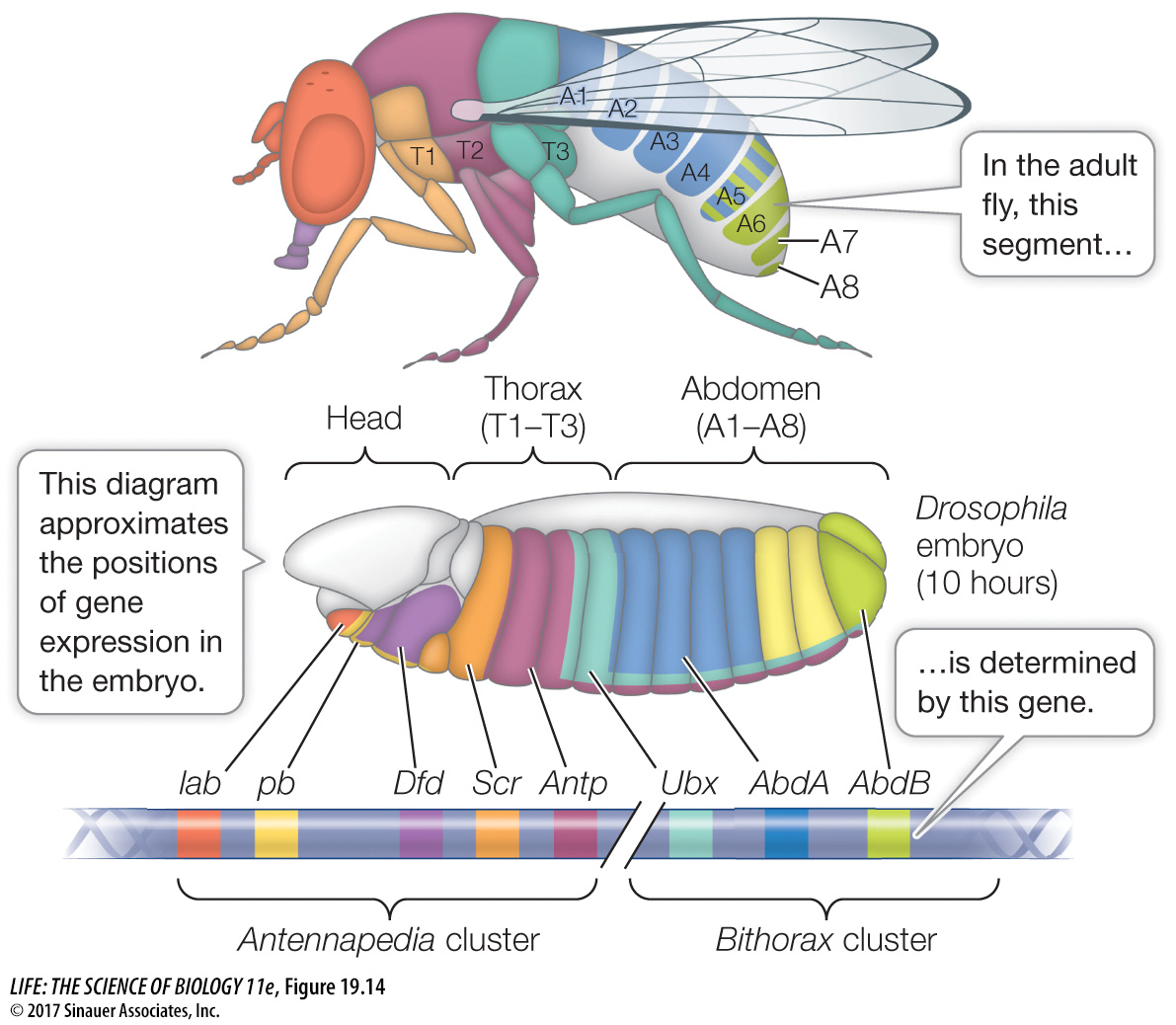
In Drosophila, the maternal effect genes, segmentation genes, and Hox genes interact to “build” a larva step by step, beginning with the unfertilized egg. How do we know that the Hox genes determine segment identity? A clue comes from homeotic mutations. A mutation in the Hox gene Antennapedia causes legs to grow on the head in place of antennae.
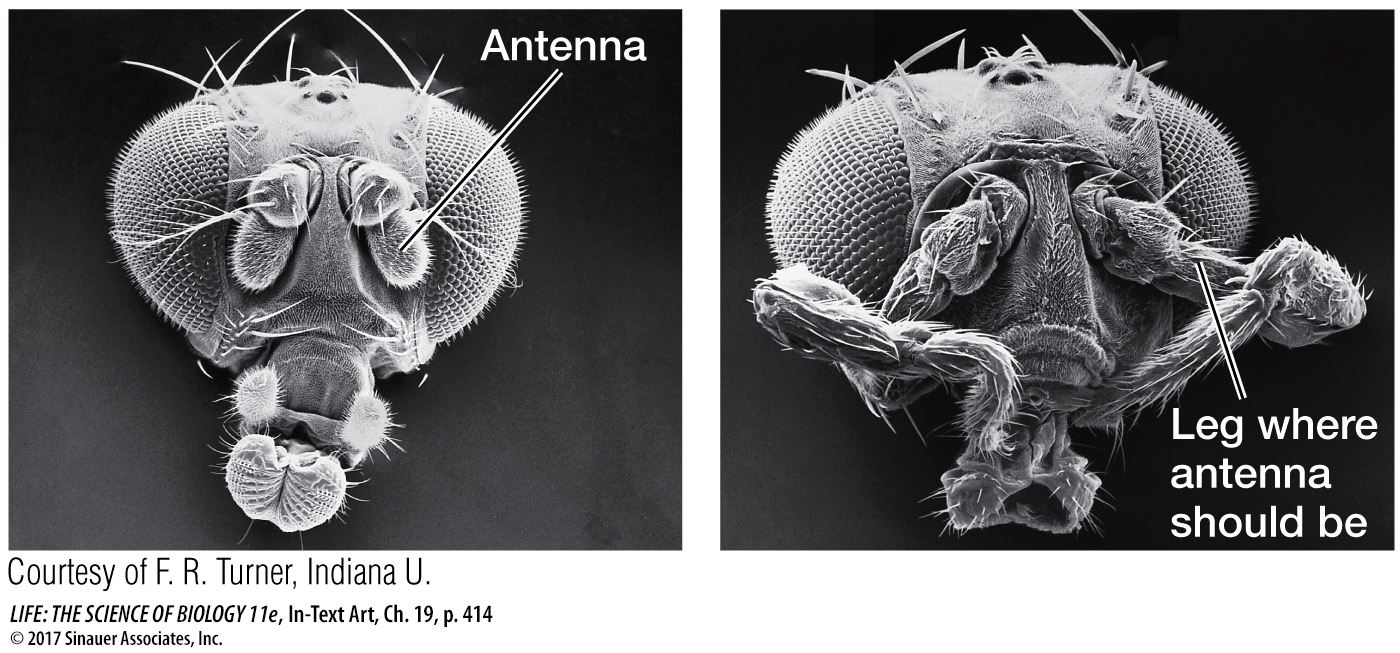
When another Hox gene—
The Antennapedia and Ultrabithorax genes both encode transcription factors and have a common 180 base-