Positive and purifying selection can be detected in the genome
As we have just seen, substitutions in a protein-
If a given amino acid in a protein can be one of many alternatives (without changing the protein’s function), then an amino acid replacement is neutral with respect to the fitness of an organism. In this case, the rates of synonymous and nonsynonymous substitutions in the corresponding DNA sequences are expected to be very similar, so the ratio of the two rates should be close to 1.
If a given amino acid position is under positive selection for change, the observed rate of nonsynonymous substitutions is expected to exceed the rate of synonymous substitutions in the corresponding DNA sequences.
If a given amino acid position is under purifying selection, then the observed rate of synonymous substitutions is expected to be much higher than the rate of nonsynonymous substitutions in the corresponding DNA sequences.
By comparing the gene sequences that encode homologous proteins from many species, scientists can determine the history and timing of synonymous and nonsynonymous substitutions. This information can be mapped on a phylogenetic tree, as we saw in Chapter 21. Regions of genes that are evolving under neutral, purifying, or positive selection can be identified by comparing the nature and rates of substitutions across the phylogenetic tree.
By examining genome sequences for synonymous and nonsynonymous substitutions, biologists have determined which amino acid positions of the surface proteins of influenza viruses are evolving under positive selection (to escape immune detection in their hosts). This information allows researchers to select viruses from current influenza epidemics that are the most likely to escape human immune detection. These viruses are the ones that are most likely to give rise to the next flu epidemic, so they are the best candidates to use in vaccine production. As we discussed in the opening story, production of successful flu vaccines has greatly lowered the impact of flu epidemics in recent decades.
A study of the evolution of lysozyme illustrates how and why particular amino acid positions might be under different modes of selection (Figure 23.6). The enzyme lysozyme (see Figure 3.9) is found in almost all animals. It is produced in the tears, saliva, and milk of mammals and in the albumen (whites) of bird eggs. Lysozyme digests the cell walls of bacteria, rupturing and killing them. As a result, it plays an important role as a first line of defense against invading bacteria. Most animals defend themselves against bacteria by digesting them, which is probably why most animals have lysozyme. Some animals also use lysozyme in the digestion of food.
experiment
Figure 23.6A Convergent Molecular Evolution
Original Paper: Stewart, C.-B et al. 1987. Adaptive evolution in the stomach lysozymes of foregut fermenters. Nature 330: 401–
Langurs (a group of monkeys) and cattle are only distantly related but both have evolved foregut fermentation. They uniquely express the enzyme lysozyme in their stomachs (foreguts) to aid in breaking down bacteria that are involved in fermentation. Stewart and colleagues compared the gene sequences of lysozyme in mammals with and without foregut fermentation to see if there is convergence in the independently evolved amino acid sequences of lysozyme in langurs and cattle.
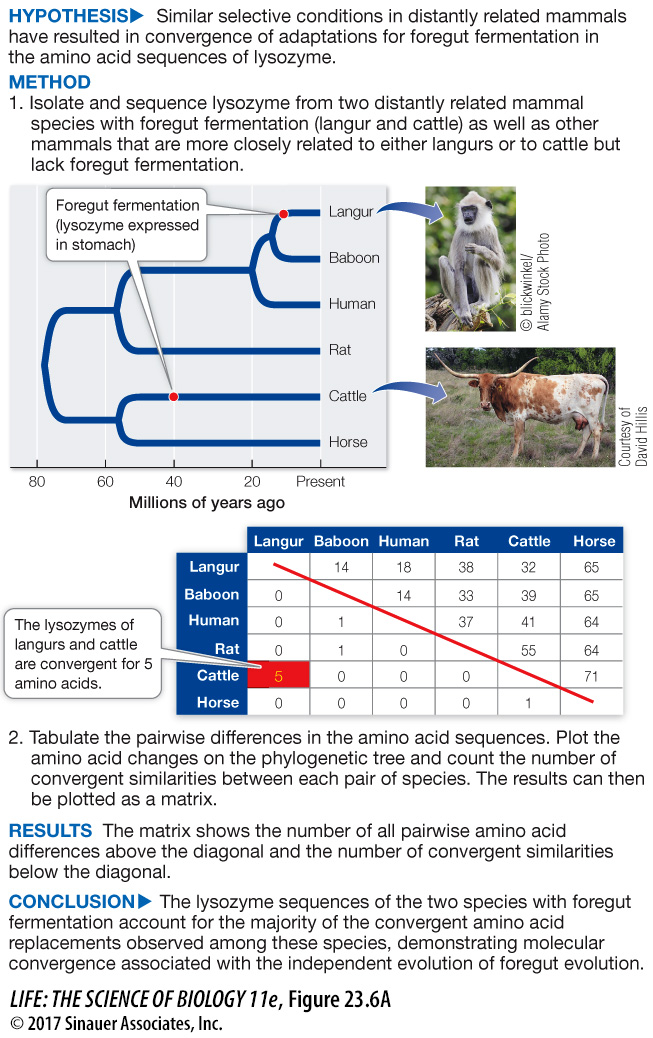
work with the data
Figure 23.6B Convergent Molecular Evolution
Original Paper: Stewart, C.-B., J. W. Schilling, and A. C. Wilson. 1987.
Caro-
| Amino acid position | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | 2 | 14 | 17 | 21 | 50 | 63 | 75 | 87 | 117 | 118 | 130 |
| Langur | I | K | L | K | E | Y | D | N | Q | N | V |
| Baboon | I | R | L | R | Q | Y | N | D | Q | N | V |
| Human | V | R | M | R | R | Y | N | D | Q | N | V |
| Rat | T | R | M | Y | Q | Y | N | D | K | N | V |
| Cattle | V | K | L | K | E | W | D | N | R | D | L |
| Horse | V | A | M | G | G | W | N | E | K | D | L |
| Ancestral state | V | R | M | R | Q | W | N | D | K | N | V |
QUESTIONS
1.
Using the phylogenetic tree (see Figure 23.6A), plot the amino acid changes across the phylogeny of the six mammals. Assume that the ancestral state is the amino acid present at the base of the tree.
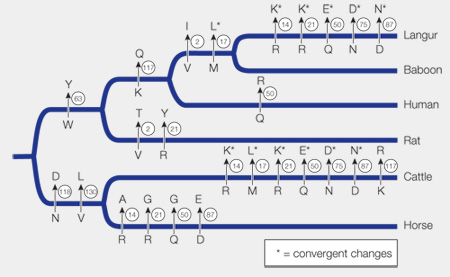
2.
Which amino acid positions show unique convergence between the langur and cattle lineages (i.e., the derived state is found only in cattle and langurs)?
Positions 14, 21, 50, 75, and 87 show convergence in amino acids between cattle and langur.
3.
Which additional position is convergent between cattle and the ancestor of langurs and baboons?
Position 17 is convergent between cattle and the ancestor of langurs and baboons.
4.
Did you detect any other convergent amino acid changes between any other pair of lineages? What does this suggest about the convergent changes you observed between cattle and langurs?
No, there are no other convergent changes. The fact that almost all convergent events occur between cattle and langurs supports the hypothesis that these convergent changes are related to the convergence in function associated with a shift to foregut fermentation.
A similar work with the data exercise may be assigned in LaunchPad.
Among mammals, a mode of digestion called foregut fermentation has evolved twice. In mammals with this mode of digestion, the foregut—
In both foregut-
For many of the amino acid positions of lysozyme, the rate of synonymous substitutions in the corresponding gene sequence was much higher than the rate of nonsynonymous substitutions. This observation indicates that many of the amino acids that make up lysozyme are evolving under purifying selection. In other words, there is selection against change in the protein at these positions, and the observed amino acids must therefore be critical for lysozyme function. At other positions, several different amino acids function equally well, and the corresponding gene sequences had similar rates of synonymous and nonsynonymous substitutions. The most striking finding was that amino acid replacements in lysozyme happened at a much higher rate in the lineage leading to langurs than in any other primate lineage. The high rate of nonsynonymous substitutions in the langur lysozyme gene shows that lysozyme went through a period of rapid change in adapting to the foregut of langurs. Moreover, the lysozymes of langurs and cattle share five unique amino acid replacements, all of which lie on the surface of the lysozyme molecule, well away from the enzyme’s active site. Two of these shared replacements involve changes from arginine to lysine, which makes the proteins more resistant to attack by the stomach enzyme pepsin. By understanding the functional significance of amino acid replacements, molecular evolutionists can explain the observed changes in amino acid sequences in terms of changes in the functioning of the protein.
A large body of fossil, morphological, and molecular evidence shows that langurs and ruminants do not share a recent common ancestor. Yet langur and ruminant lysozymes share several amino acids that neither mammal shares with the lysozymes of its own closer relatives. The lysozymes of these two mammals have undergone convergent evolution at some amino acid positions despite their very different ancestry. The amino acids they share give these lysozymes the ability to lyse the bacteria that ferment plant material in the foregut.
The hoatzin, an unusual leaf-