Radioactive isotopes of atoms—*radioisotopes—decay in a predictable pattern over long periods. Over a specific time interval, known as a half-life, half of the atoms in a radioisotope decay to become a different isotope (Figure 24.1A). The use of this knowledge to date fossils and rocks is known as radiometric dating.
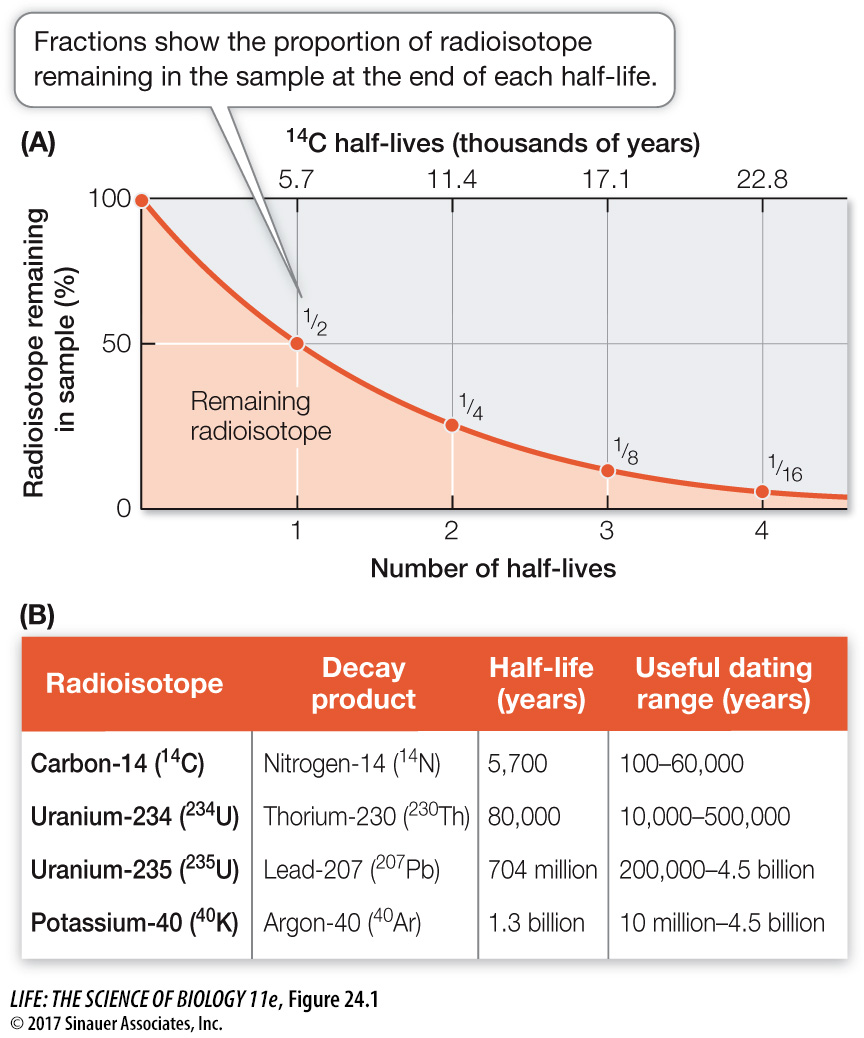
Figure 24.1 Radioactive Isotopes Allow Us to Date Ancient Rocks The decay of radioactive isotopes into stable isotopes happens at a steady rate. A half-life is the time it takes for half of the remaining atoms to decay in this way. (A) The graph demonstrates the principle of half-life using carbon-14 (14C) as an example. The half-life of 14C is 5,700 years. (B) Different radioisotopes have different characteristic half-lives that allow us to estimate the ages of many rocks.
To use a radioisotope to date a past event, we must know or estimate the concentration of that isotope at the time of that event, and we must know the radioisotope’s half-life. In the case of carbon-14, a radioisotope of carbon, the production of new carbon-14 (14C) in the upper atmosphere—by the reaction of neutrons with nitrogen-14 (14N, a stable isotope of nitrogen)—just balances the natural radioactive decay of 14C into 14N. Therefore the ratio of 14C to the more common stable isotope of carbon, carbon-12 (12C), is relatively constant in living organisms and in their environment. As soon as an organism dies, however, it ceases to exchange carbon compounds with its environment. Its decaying 14C is no longer replenished, and the ratio of 14C to 12C in its remains decreases over time. Paleontologists can use the ratio of 14C to 12C in fossil material to date fossils that are less than 60,000 years old (and thus the sedimentary rocks that contain those fossils). If fossils are older than that, so little 14C remains that the limits of detection using this particular isotope are reached, and other radioisotopes must be used instead.
