Nitrogenase catalyzes nitrogen fixation
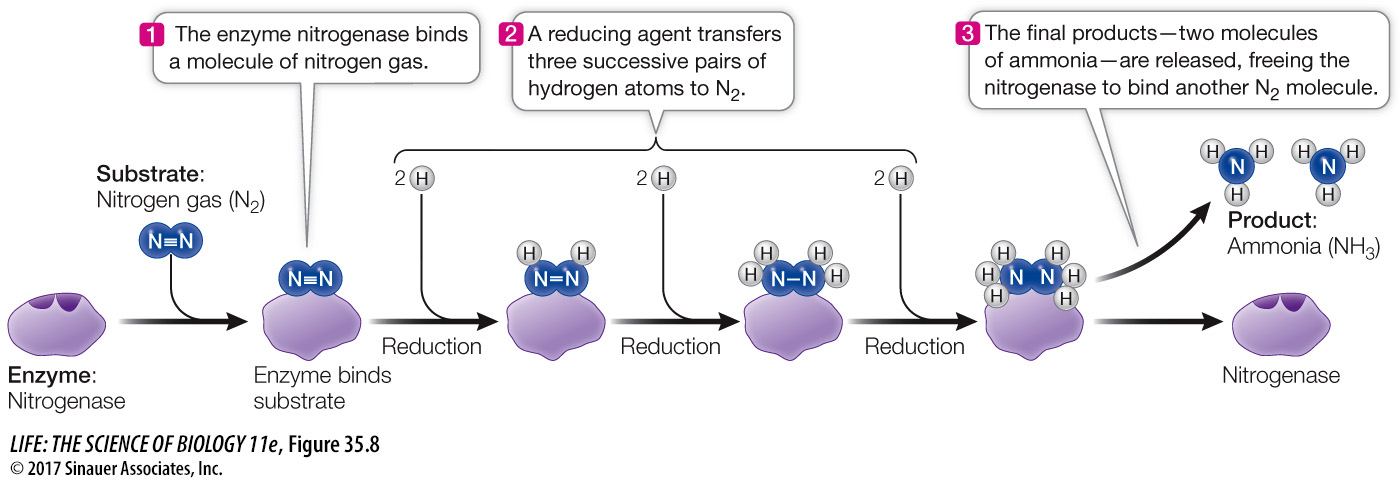
Nitrogen fixation is the reduction (see Key Concept 9.1) of nitrogen gas. It proceeds by the stepwise addition of three pairs of hydrogen atoms to N2 (Figure 35.8). In addition to N2, these reactions require three things:
A strong reducing agent (e.g., ferredoxin) to transfer hydrogen atoms (protons and electrons) to N2 and to the intermediate products of the reaction
A great deal of energy, which is supplied by ATP
The enzyme nitrogenase, which catalyzes the reaction
Q: Why does O2 inhibit this reaction?
O2 is a strong oxidizing agent, and could attract electrons (and hydrogen atoms) that are needed for nitrogenase. These electrons come from a strong reducing agent.
Nitrogenase is strongly inhibited by oxygen, and many nitrogen fixers are anaerobes that live in environments with little or no O2. But rhizobia are aerobic and fix nitrogen in aerobic plant roots. How can nitrogenase function under these circumstances?
Plants typically house nitrogen-
investigating life
Mycorrhizal Fungi Can Replace Fertilizer in Cassava Cultivation
experiment
Original Paper: Ceballos, I., M. Ruiz, C. Fernandez, R. Pena, A. Rodriguez and I. Sanders. 2013. The in vitro mass-
Phosphorous is a plant macronutrient obtained from the soil. Unfortunately, in many tropical regions the phosphate level in the soil is inadequate to support the needs of crop production, forcing farmers to use inorganic phosphate fertilizer, which is a finite and expensive resource. The need for phosphate fertilizer poses an especially acute problem for farmers in poor, tropical regions of the world.
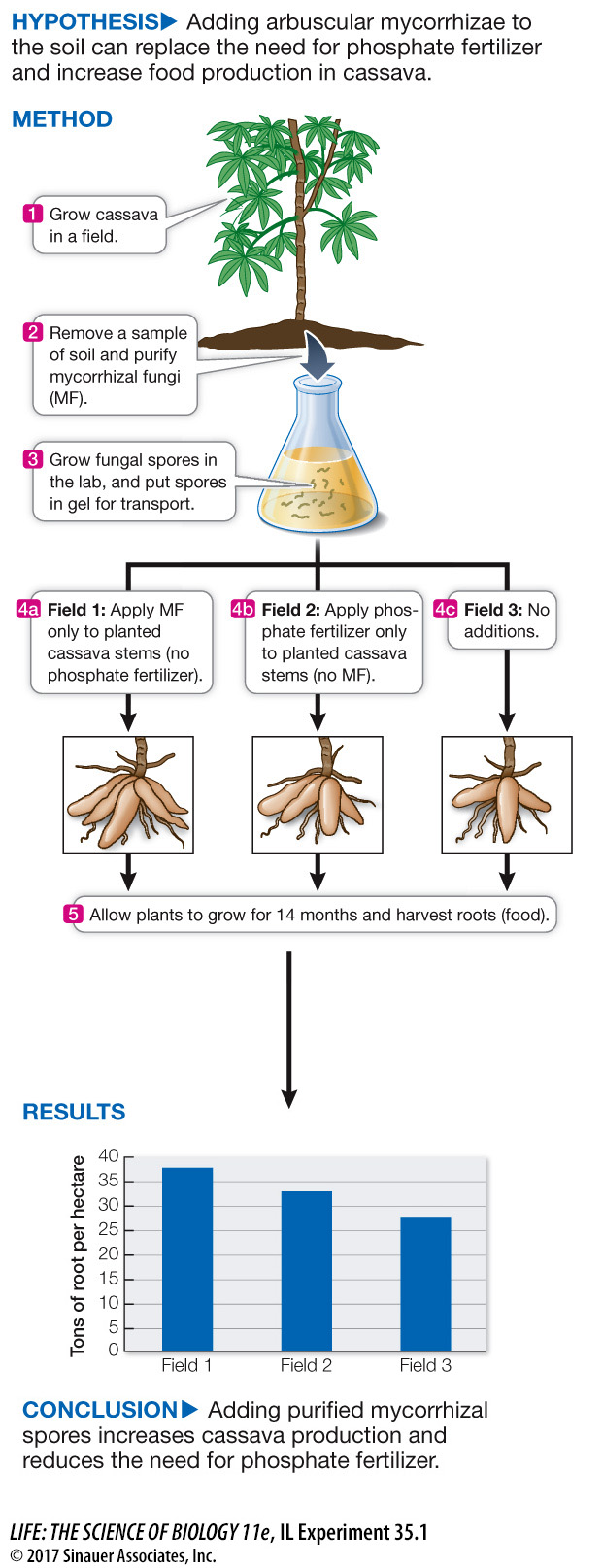
Mycorrhizal fungi very efficiently absorb phosphate ions from the soil. Plant roots with a symbiotic relationship with mycorrhizae obtain phosphorous, while the fungi obtain photosynthate (products of photosynthesis) from the plant. Ian Sanders and his team at the University of Lausanne in Switzerland, in collaboration with Alia Rodriguez and her team at the National University of Colombia in Bogota, investigated whether production of cassava could be increased by adding concentrated spores of mycorrhizae, thus lowering the need for phosphate fertilizer.
work with the data
QUESTIONS
1.
Concentrated spores of the mycorrhizal fungus Rhizophagus irregularis were added to fields of cassava stems tied to stakes. Control fields did not receive the spores. The experiments took place at Yopal, Colombia, in a tropical region. At various times, developing roots were examined microscopically for the presence of fungal hyphae. The results are shown in Table A. Storage roots were harvested after 400 days.
| Days after spores were added | Percent colonization |
|---|---|
| 45 | 40 |
| 90 | 45 |
| 135 | 47 |
| 180 | 49 |
| 225 | 40 |
| 270 | 62 |
| 315 | 82 |
Plot the data as percent colonization versus time (days after spores added). When did the colonization of fungi increase? Why do you think there was a lag time?
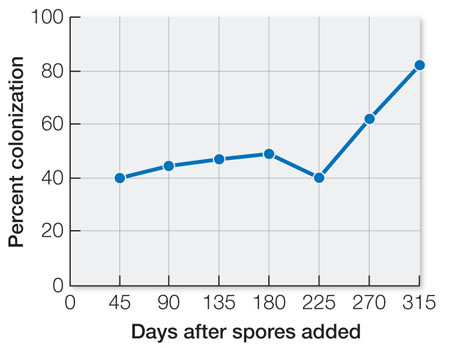
Root colonization was maximal 315 days after spores were added. The lag time was probably due to the need for the spores to germinate and grow into hyphae that could colonize the cassava roots.
2.
In a large field experiment, plots of 300 plants each were treated either with or without fungal spores, and with or without phosphate fertilizer in an amount usually needed for optimal growth (100%) or half of that (50%). The results are shown in Table B, as fresh weight (g) of cassava roots, the crop (±SEM).
| No fertilizer | 50% fertilizer | 100% fertilizer | |
|---|---|---|---|
| Fungal spores | 38 (2) | 40 (1) | 43 (2) |
| No fungal spores | 28 (2) | 36 (3) | 35 (2) |
Did the addition of mycorrhizal spores affect the cassava production? What is the evidence for your answer?
What statistical test would you use to test for significance of the effects of fertilizer and added mycorrhizae?
- Cassava root crop production was just as good with fungal spores alone (38 g) as with fertilizer alone (35 g). Adding spores to fertilizer had a positive effect (43 g).
- A t-test for paired samples could be used to test for significance in differences for spores-
treated and untreated experiments.
A similar work with the data exercise may be assigned in LaunchPad.