Plants produce constitutive chemical defenses against herbivores
Plants attract, resist, and inhibit other organisms with a wide range of chemicals known as secondary metabolites. Primary metabolites are substances such as proteins, nucleic acids, carbohydrates, lipids, and their building blocks, which are produced and used by all living organisms, including plants. Primary metabolites are used in basic cellular processes such as photosynthesis, respiration, and nutrient uptake. Secondary metabolites are substances that are not used for basic cellular processes. Each is found in only certain organisms or groups of organisms.
The more than 10,000 known plant secondary metabolites range in molecular mass from about 70 to more than 380,000 daltons, but most have a low molecular mass (Table 38.1). The effects of defensive secondary metabolites on animals are diverse. Some act on the nervous systems of herbivorous insects, mollusks, or mammals. Others mimic the natural hormones of insects, causing some larvae to fail to develop into adults. Still others damage the digestive tracts of herbivores. Some secondary metabolites are toxic to fungal pathogens. Many secondary metabolites are not just used for defense; they have other roles in the plant. For instance, the phenolics called anthocyanins are toxic to insects and bacteria. But they also are pigmented and aid in attracting pollinators, accumulate in vacuoles and so contribute to osmotic pressure in cells, and even regulate dormancy in some plants.
| Class | Type | Role | Example |
|---|---|---|---|
|
Nitrogen- 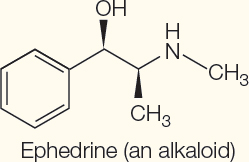
|
Alkaloids | Neurotoxin | Nicotine in tobacco |
| Glycosides | Inhibit electron transport | Dhurrin in sorghum | |
| Nonprotein amino acids | Disrupt protein structure | Canavanine in jack bean | |
|
Nitrogen– 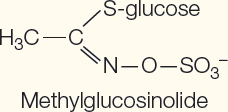
|
Glucosinolates | Inhibit respiration | Methylglucosinolate in cabbage |
|
Phenolics 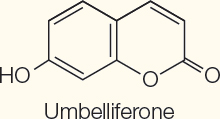
|
Coumarins | Block cell division | Umbelliferone in carrots |
| Flavonoids | Phytoalexins | Capsidol in peppers | |
| Tannins | Inhibit enzymes | Gallotannin in oak trees | |
|
Terpenes 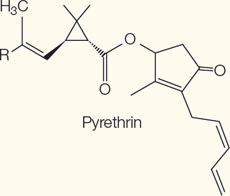
|
Monoterpenes | Neurotoxins | Pyrethrin in chrysanthemums |
| Diterpenes | Disrupt reproduction and muscle function | Gossypol in cotton | |
| Triterpenes | Inhibit transport | Digitalis in foxglove | |
| Sterols | Block animal hormones | Spinasterol in spinach | |
| Polyterpenes | Deter feeding | Latex in Euphorbia |
Here is an example of how one of these metabolites works on pathogens. Canavanine is a secondary metabolite whose role is defensive and is based on its chemical structure. Canavanine is an amino acid that is not normally found in proteins, but is very similar to the amino acid arginine, which is found in almost all proteins.
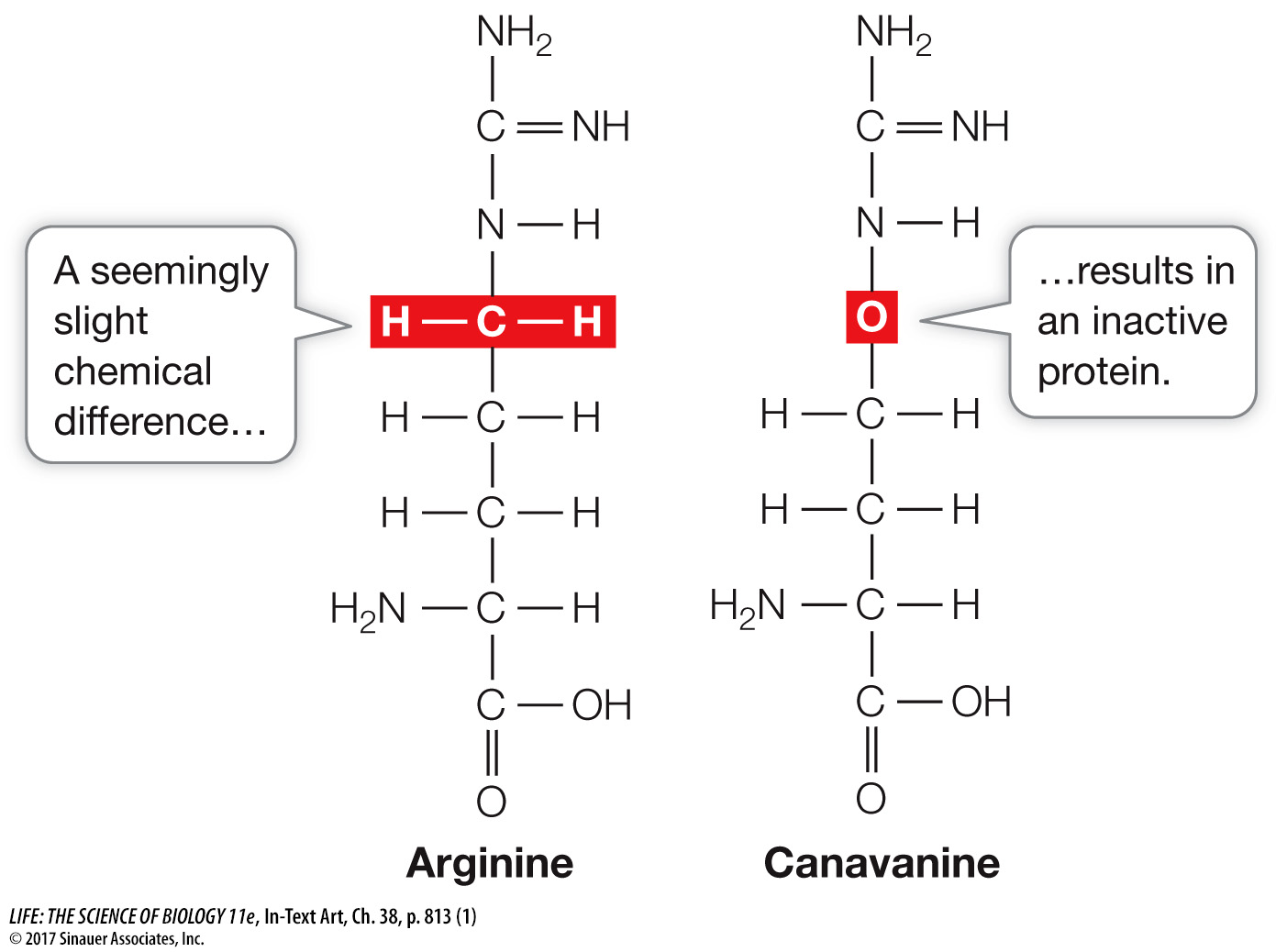
When an insect larva consumes canavanine-
Taking cues from nature, we have practical uses for many plant secondary metabolites or molecules made from them in the lab. Throughout human history, these have formed the basis of herbal medicine as plant extracts are used. As knowledge of chemistry accumulated, people purified the active ingredients of these plants. For instance, the foxglove plant gave us the cardiac drug digitalis.