Insulin and glucagon regulate blood glucose concentrations
Before the 1920s, the disease diabetes mellitus was fatal. Characterized by weakness, lethargy, and a dramatic loss of body mass, this condition was known to be connected somehow with the pancreas—
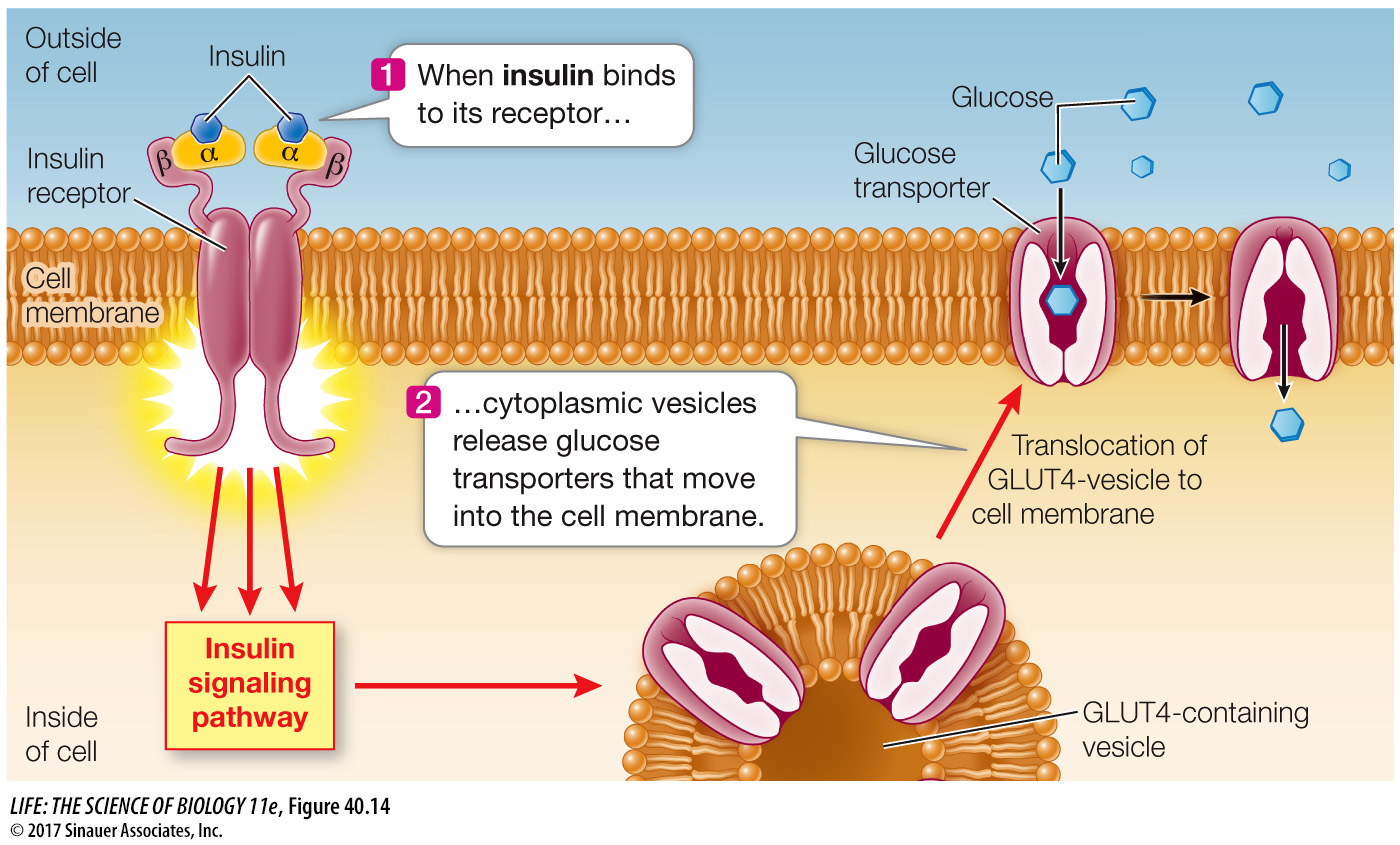
Today we know there are two forms of diabetes mellitus. The form that was mostly seen prior to the 1920s usually occurred in young people and was called juvenile diabetes. We now call it type I diabetes, and it is caused by a lack of the protein hormone insulin. Glucose enters cells by diffusion, but cell membranes are not very permeable to glucose. Glucose transporter proteins in cell membranes facilitate the movement of glucose into cells, and the glucose transporters called GLUT4 that are most common in muscle and adipose tissue are controlled by insulin. When insulin binds to its receptor on the cell membrane, it causes these glucose transporters to move from cytoplasmic vesicles to the cell membrane, thus making the cell more permeable to glucose (Figure 40.14). When insulin is not present, these transporters are returned to the cytoplasmic pool through endocytosis.
The other form of diabetes, which is seen in epidemic proportions in the United States today, is called adult onset or type II diabetes. Type II diabetes is associated with obesity, high carbohydrate consumption, and lack of exercise. It is caused either by low production of insulin or by insensitivity to insulin. More than 90 percent of diabetes cases seen in the United States today are type II diabetes, and the numbers are rising alarmingly. Statistics from the Centers for Disease Control and Prevention show that the number of new cases of type II diabetes reported in the United States in 1991 was less than 600,000 and had been fairly stable since 1980. Between 1991 and 2012, the annual number of new cases tripled. Diabetes is currently the seventh leading cause of death in the United States, and it is a common contributor to blindness, kidney failure, stroke, heart disease, and amputations resulting from ulcerations. This disease is largely preventable and even reversible with lifestyle changes.
In both type I and type II diabetes, glucose entry into cells is impaired, resulting in so much glucose accumulating in the blood that it starts to spill over into the urine. A high concentration of glucose in the blood increases urine output by two mechanisms. First, it causes water to move from cells into the blood by osmosis, and this increase in blood volume results in increased urine production. Second, the increased glucose in the tubules of the kidneys pulls more water into the urine by osmosis. Diabetic individuals thus lack metabolic fuel, and they can also become dehydrated. Because glucose uptake by muscle and adipose tissue is impaired in the absence of insulin, muscle cells must depend on fat and protein for fuel and adipose tissue cannot replenish its stores of triglycerides. If the condition is not treated, the body wastes away.
For centuries, the prospects for people suffering with type I diabetes were bleak. A change came almost overnight in 1921, when the physician Frederick Banting and a medical student, Charles Best, at the University of Toronto, discovered they could reduce the symptoms of diabetes by injecting an extract prepared from pancreatic tissue. The active component of this extract was found to be insulin, a small protein consisting of just 51 amino acids. In the United States today, insulin replacement therapy using manufactured insulin allows more than 1.5 million people with type I diabetes to lead almost normal lives.
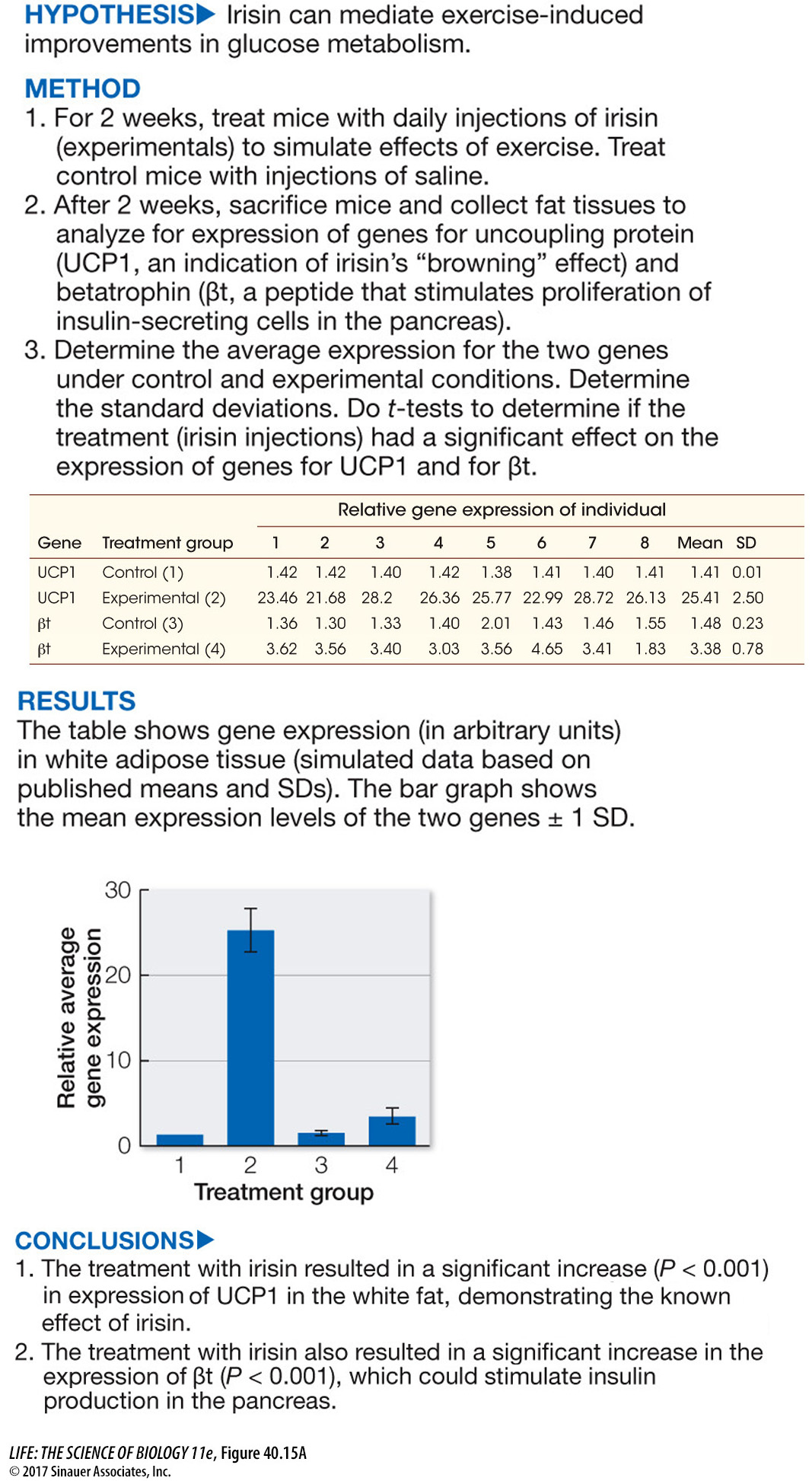
Much evidence now shows that type II diabetes can be treated with a combination of dietary changes and weight loss, and Figure 40.15 looks at the intriguing question of whether the hormone irisin (introduced in the opening of this chapter) can mediate the beneficial effects of exercise on glucose metabolism in type II diabetes patients.
experiment
Figure 40.15A Effects of Exercise on Glucose Metabolism
Original Paper: Zhang, Y. et al. 2014. Irisin stimulates browning of white adipocytes through mitogen-
Exercise has been shown to have benefits for individuals with type II diabetes and for obese individuals. Imaging studies have revealed an inverse correlation between brown fat abundance and body mass—
work with the data
Figure 40.15B Effects of Exercise on Glucose Metabolism
Original Paper: Zhang et al. 2014.
Figure 40.15A showed that elevated irisin levels could stimulate pancreatic function in ways that could benefit individuals with type II diabetes. Betatrophin had been shown to stimulate proliferation of insulin-
| Group | Body mass (g) before |
Body mass (g) after |
Fasting insulin (ng/mL) |
Blood glucose (mg/dL) |
|---|---|---|---|---|
| Control | 40.1 ± 4.4 | 40.9 ± 4.8 | 4.3 ± 1.0 | 150 ± 5 |
| Irisin treated | 43.7 ± 2.7 | 40.2 ± 2.8 | 2.1 ± 0.5 | 130 ± 7 |
QUESTIONS
1.
In the first experiment, why was it important to measure a known marker of brown fat function, namely UCP1, in the same experiment in which the expression of a new candidate gene, namely βt was being measured as a response to irisin treatment?
In the experiment in which irisin was injected into the mice kept on a high-
2.
In the second experiment, why were the mice first placed on a high-
The mice were placed on a high-
3.
Type II diabetes is frequently associated with obesity and is characterized by insulin resistance. As a result, insulin levels can be high while glucose metabolism is still impaired. What evidence was produced supporting the hypothesis that raised irisin levels could benefit obese individuals or individuals with type II diabetes?
The data support the hypothesis that irisin could reduce obesity and lower the risk for type II diabetes for the following reasons. Using t-tests confirms that the two groups of mice prior to irisin application did not differ in body mass. However, the irisin-
A similar work with the data exercise may be assigned in LaunchPad.
ISLETS OF LANGERHANS Insulin is produced in clusters of endocrine cells in the pancreas. These clusters are called islets of Langerhans after the German medical student who discovered them. They contain three types of cells, each of which produces a specific hormone:
Beta (β) cells produce and secrete insulin.
Alpha (α) cells produce and secrete glucagon, a hormone that has effects mostly opposite from those of insulin.
Delta (δ) cells produce the hormone somatostatin.
The rest of the pancreas is made up of exocrine tissue, which produces enzymes and other secretions that travel through ducts to the gut, where they participate in digestion.
After a meal, the concentration of glucose in the blood rises, stimulating the β cells of the islets to release insulin. Insulin causes target cells throughout the body to use circulating glucose as fuel and convert it into storage products such as glycogen and fat. When the gut is empty of food, blood glucose concentration falls and the islets stop releasing insulin. As a result, most cells shift to using glycogen and fat rather than glucose as fuel. If blood glucose concentration falls substantially below normal, the islet α cells release glucagon, which stimulates the liver to break down stored glycogen and release glucose into the blood. We will discuss these actions in greater detail in Key Concept 50.4.
SOMATOSTATIN Somatostatin is released from the δ cells of the pancreas in response to rapid increases of glucose and amino acids in the blood. This hormone has paracrine functions within the islets, where it inhibits the release of both insulin and glucagon. Outside the pancreas it acts as a hormone, slowing the digestive activities of the gut and extending the period during which nutrients are absorbed. Somatostatin is also produced in very small amounts by cells in the hypothalamus. Hypothalamic somatostatin, also called growth hormone inhibiting hormone (GHIH), is transported in the portal blood vessels to the anterior pituitary, where it inhibits the release of growth hormone and thyrotropin.