Printed Page 880
There are five classes of immunoglobulins
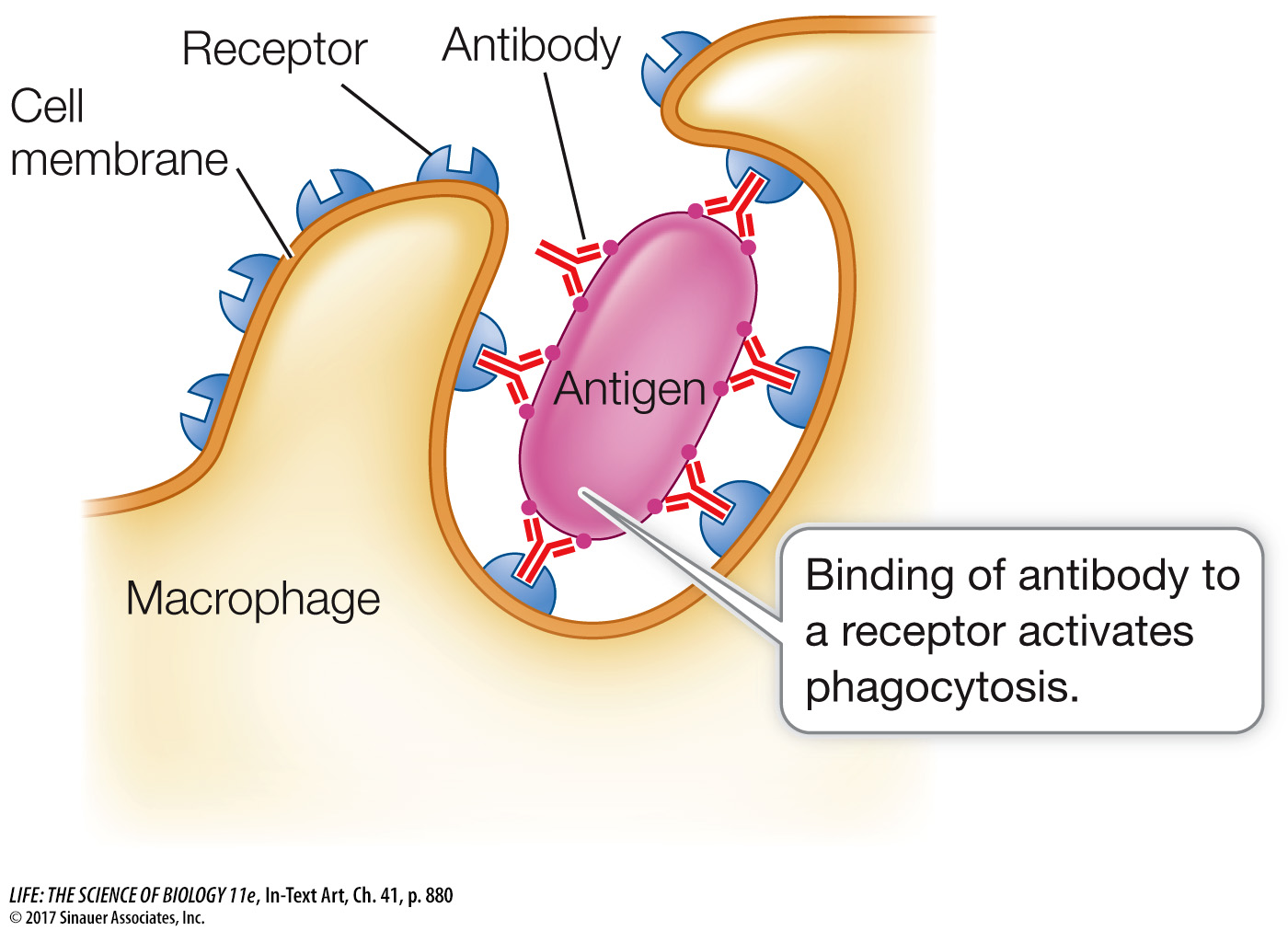
While the variable regions are responsible for the specificity of an immunoglobulin, the constant regions of the heavy chain determine the class of the immunoglobulin. The five immunoglobulin classes are described in Table 41.2. The most abundant class is IgG; these soluble antibody proteins make up about 80 percent of the total immunoglobulin content of the bloodstream. They are made in greatest quantity during a secondary immune response. IgG molecules defend the body in several ways. For example, after some IgG molecules bind to antigens, they become attached by their heavy chains to macrophages. This attachment permits the macrophages to destroy the antigens by phagocytosis.
table 41.2 Antibody Classes
| Class | General structure | Location | Function | |
|---|---|---|---|---|
| IgG | Monomer |

|
Free in blood plasma; about 80 percent of circulating antibodies | Most abundant antibody in primary and secondary immune responses; crosses placenta and provides passive immunization to fetus |
| IgM | Pentamer |

|
Surface of B cell; free in blood plasma | Antigen receptor on B cell membrane; first class of antibodies released by B cells during primary response |
| IgD | Monomer |

|
Surface of B cell | Cell surface receptor of mature B cell; important in B cell activation |
| IgA | Dimer |
|
Saliva, tears, milk, and other body secretions | Protects mucosal surfaces; prevents attachment of pathogens to epithelial cells |
| IgE | Monomer |

|
Secreted by plasma cells in skin and tissues lining gastrointestinal and respiratory tracts | Binds to mast cells and basophils to sensitize them to subsequent binding of antigen, which triggers release of histamine that contributes to inflammation and some allergic responses |