The dorsal lip of the blastopore organizes the formation of the amphibian embryo
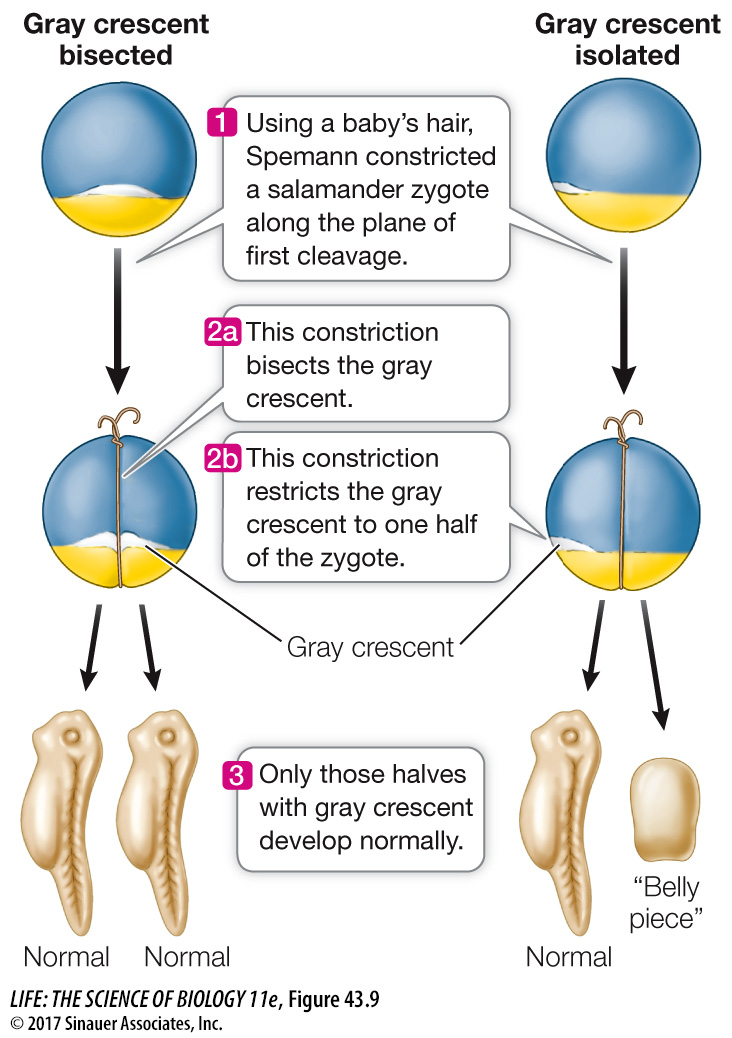
In the early 1900s the German biologist Hans Spemann was studying the development of salamander eggs. He was interested in finding out whether the nuclei of blastomeres remain capable of directing the development of complete embryos or whether these nuclei lose some developmental potential. With great patience and dexterity, he formed loops from single hairs taken from a baby (in fact, his daughter) and tied them around fertilized eggs along the plane of the first cell division, effectively dividing the eggs in half, with the nucleus restricted to one side. The side containing the nucleus went through cell divisions and developed into a salamander; the other half simply degenerated. Up until the 16-
As often happens in science, Spemann’s bisection experiments revealed a new phenomenon. Sometimes the half of the blastula receiving an escaped nucleus did not develop. When his loops bisected the gray crescent, both halves of the zygote developed into a complete embryo. When he tied the loops so the gray crescent was on only one side of the constriction, however, only that half of the zygote developed into a complete embryo (Figure 43.9). When the half lacking gray crescent material received a nucleus, it underwent cell division but formed only a clump of undifferentiated cells that Spemann called a “belly piece.” Spemann hypothesized that cytoplasmic factors unequally distributed in the fertilized egg were necessary for gastrulation and the development of a normal salamander.
To further test the hypothesis that cells receiving different complements of cytoplasmic factors had different developmental fates, Spemann transplanted pieces of early gastrulas to various locations on other gastrulas. Guided by fate maps (see Figure 43.6), he was able to take a piece of ectoderm he knew would develop into the epidermis of the skin and transplant it to a region that normally becomes part of the nervous system, and vice versa.
When he performed these transplants in early gastrulas—
In late gastrulas, however, the same experiment yielded opposite results. Transplanted cells destined to become epidermis in their original location produced patches of skin cells in the host nervous system, and the transplanted cells from regions that would develop into nervous system tissue produced nervous tissue in the skin of the recipient. At some point during gastrulation, the fates of the embryonic cells had become determined.
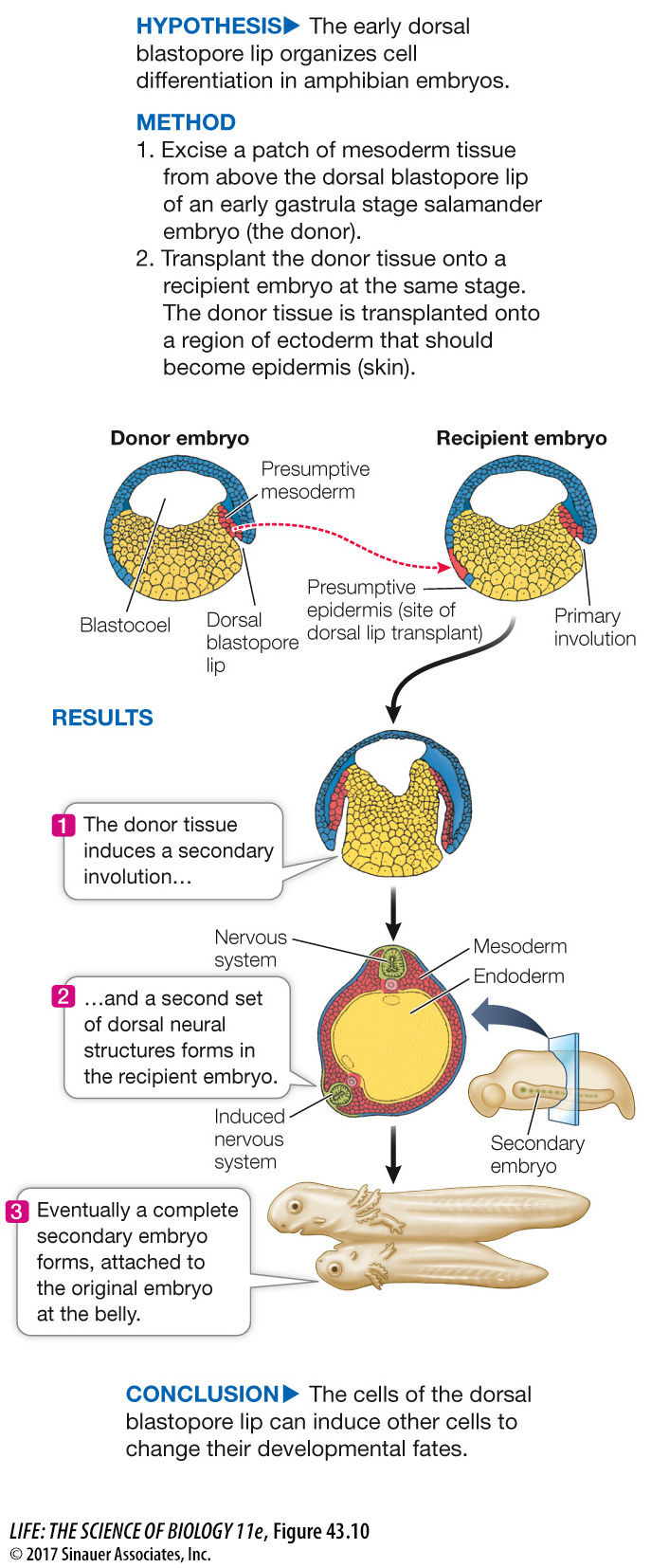
Spemann’s next experiment, done with his student Hilde Mangold, produced momentous results: they transplanted the dorsal lip of the blastopore (Figure 43.10). When this small piece of tissue was transplanted into the presumptive belly area of another gastrula, it stimulated a second site of gastrulation—
experiment
Figure 43.10 The Dorsal Lip Induces Embryonic Organization
Original Paper: Spemann, H. and H. Mangold. 1924. Roux’ Arch. Entw. Mech. 100: 599–
In a classic experiment, Hans Spemann and Hilde Mangold transplanted the dorsal blastopore lip mesoderm of an early gastrula stage salamander embryo. The results showed that the cells of this embryonic region, which they dubbed “the organizer,” could direct the formation of an entire embryo.
Animation 43.2 The Primary Embryonic Organizer