Opsins are the universal photoreceptor molecule in animals
Photosensitivity in animals depends on the ability of opsin molecules to absorb photons of light and activate a G protein. Opsins are proteins that are not themselves photosensitive, but they contain covalently bound functional groups called 11-cis-retinal (Figure 45.15). Retinal absorbs photons of light, and the color of light it is receives depends on the structure of its opsin. There are several different opsins, and the most common one that gives humans sensitivity to low levels of light, but not color, is called rhodopsin.
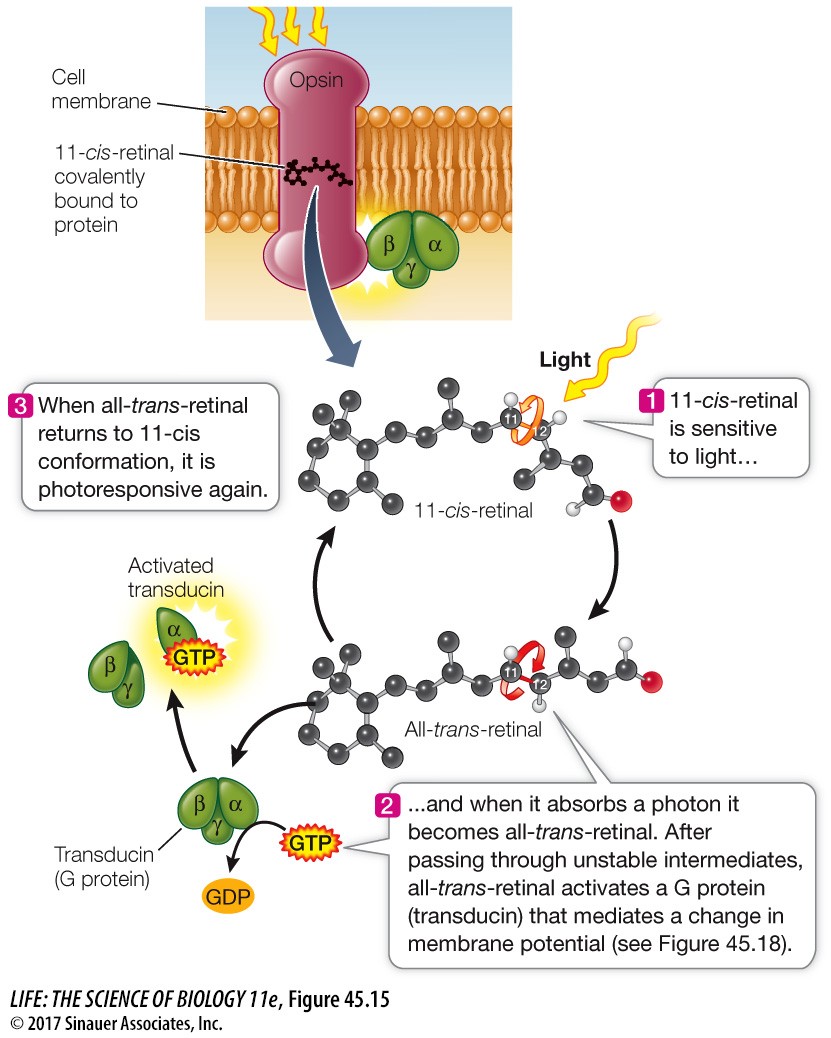
Figure 45.15 Light Changes the Conformation of Rhodopsin The light-absorbing molecule 11-cis-retinal bonds with the protein opsin to form the vertebrate visual pigment rhodopsin.
Page 975
When 11-cis-retinal absorbs a photon of light energy, it changes into a different isomer of retinal, called all-trans-retinal. This change puts a strain on the bonds between retinal and opsin, changing the conformation of opsin and signaling the detection of light. In vertebrate eyes, the retinal and the opsin eventually separate from each other (a process called bleaching), which causes the opsin to lose its photosensitivity. A series of enzymatic reactions returns the all-trans-retinal to the 11-cis isomer, which then recombines with opsin so that it once again becomes photosensitive.
How does the conformational change of opsin transduce light into a cellular response? After retinal is converted from the 11-cis to the all-trans form, its interactions with opsin pass through several unstable intermediate stages. One of these stages triggers a cascade of reactions involving a G protein signaling mechanism that results in the alteration of membrane potential that is the photoreceptor cell’s response to light.
