Actin–myosin interactions cause filaments to slide
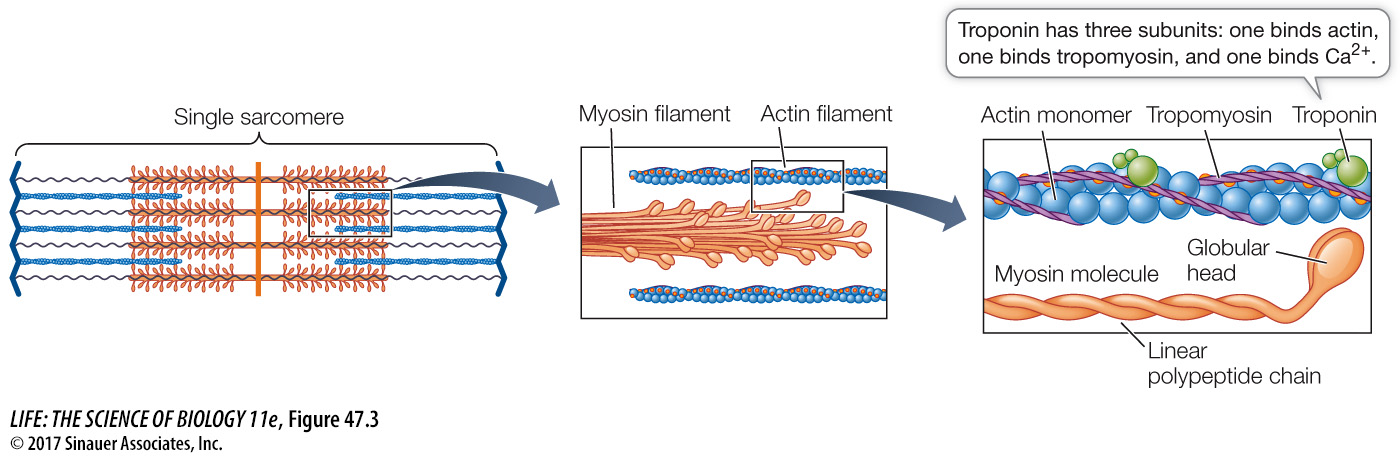
To understand the mechanism causing the actin and myosin filaments to slide past each other, we must first examine the structures of actin and myosin (Figure 47.3). A myosin molecule consists of two long polypeptide chains coiled together, each ending in a large globular head. A myosin filament is made up of many myosin molecules arranged in parallel, with their heads projecting sideways at each end of the filament like a bunch of golf clubs.
Animation 47.1 Molecular Mechanisms of Muscle Contraction
An actin filament consists of actin monomers polymerized into long chains that look like two strands of beads twisted together. Twisting around the actin chains is another protein, tropomyosin, and attached to tropomyosin at intervals are molecules of troponin. We’ll discuss these two proteins in more detail later in this section.
The myosin heads can bind specific sites on actin, to form cross-
Together these details explain the cycle of events that cause the actin and myosin filaments to slide past each other and shorten the sarcomere. They also explain rigor mortis—
We have been discussing the cycle of contraction in terms of a single myosin head. Remember that each myosin filament has many myosin heads at both ends and is surrounded by six actin filaments; thus the contraction of the sarcomere involves a great many cycles of interaction between actin and myosin molecules. That is why when a single myosin head breaks its contact with actin, the actin filaments do not slip backward.