Hemoglobin combines reversibly with O2
RBCs contain enormous numbers of hemoglobin molecules. Hemoglobin is a protein consisting of four polypeptide subunits, each of which surrounds a heme group—
Hemoglobin’s ability to pick up or release O2 depends on the PO2 in its environment. When the PO2 of the blood plasma is high, as it usually is in the lung capillaries, each hemoglobin molecule can carry its maximum load of four O2 molecules. As the blood circulates through the rest of the body, it releases some of the O2 it carries when it encounters lower PO2 values in tissues.
The relationship between PO2 and the amount of O2 bound to hemoglobin is not linear but S-
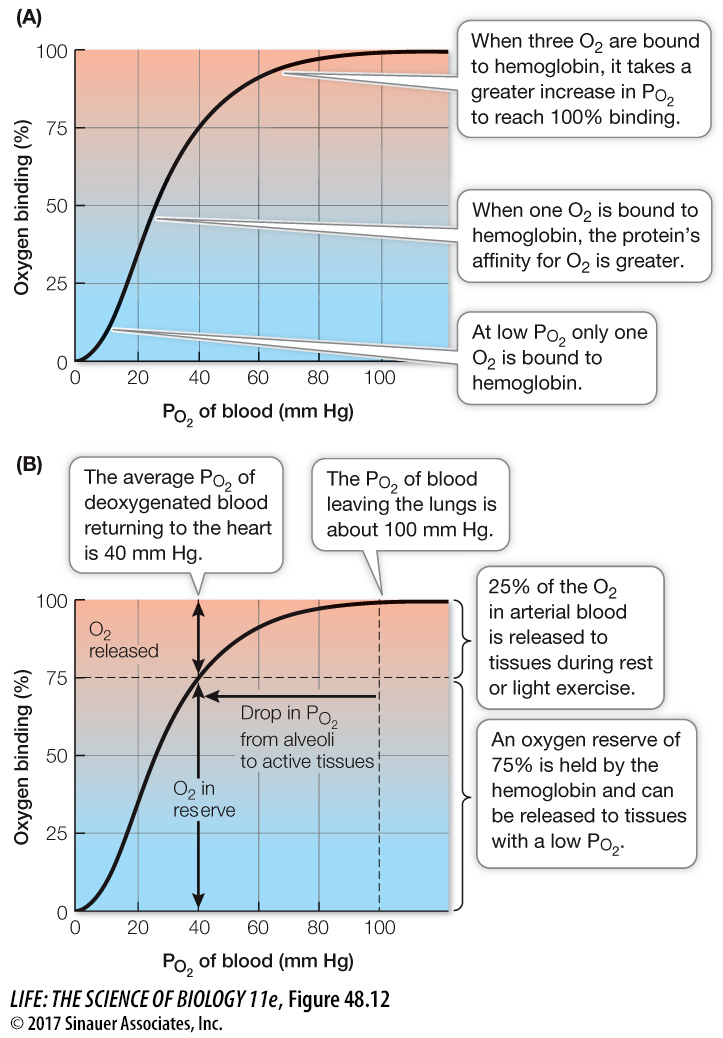
Once the third O2 molecule is bound, the relationship seems to change, as a larger increase in PO2 is required for the hemoglobin to reach 100 percent saturation. This upper bend of the sigmoid curve is due to a probability phenomenon. The closer we get to having all subunits occupied, the less likely it is that any particular O2 molecule will find a place to bind. Therefore it takes a relatively greater PO2 to achieve 100 percent saturation.
The O2-binding/dissociation properties of hemoglobin help get O2 to the tissues that need it most (Figure 48.12B). In the lungs, where the PO2 is about 100 mm Hg, hemoglobin is 100 percent saturated. The PO2 in blood returning to the heart from the body (at rest) is usually about 40 mm Hg. You can see that at this PO2 the hemoglobin is still about 75 percent saturated. This means that as the blood circulates around the body, it releases only about one in four of the O2 molecules it carries. This system seems inefficient, but because the hemoglobin keeps 75 percent of its O2 in reserve, it can meet the demands of highly active tissues.
When a tissue becomes starved of O2 and its local PO2 falls below 40 mm Hg, the hemoglobin flowing through that tissue is on the steep portion of its binding/dissociation curve. That means relatively small decreases in PO2 below 40 mm Hg will result in the release of lots of O2 to the tissue. Thus hemoglobin is very effective in making O2 available to tissues precisely when and where it is needed most.
The O2 transport function of hemoglobin can rapidly and tragically be disrupted by a common by-
Activity 48.2 Hemoglobin Loading Simulation