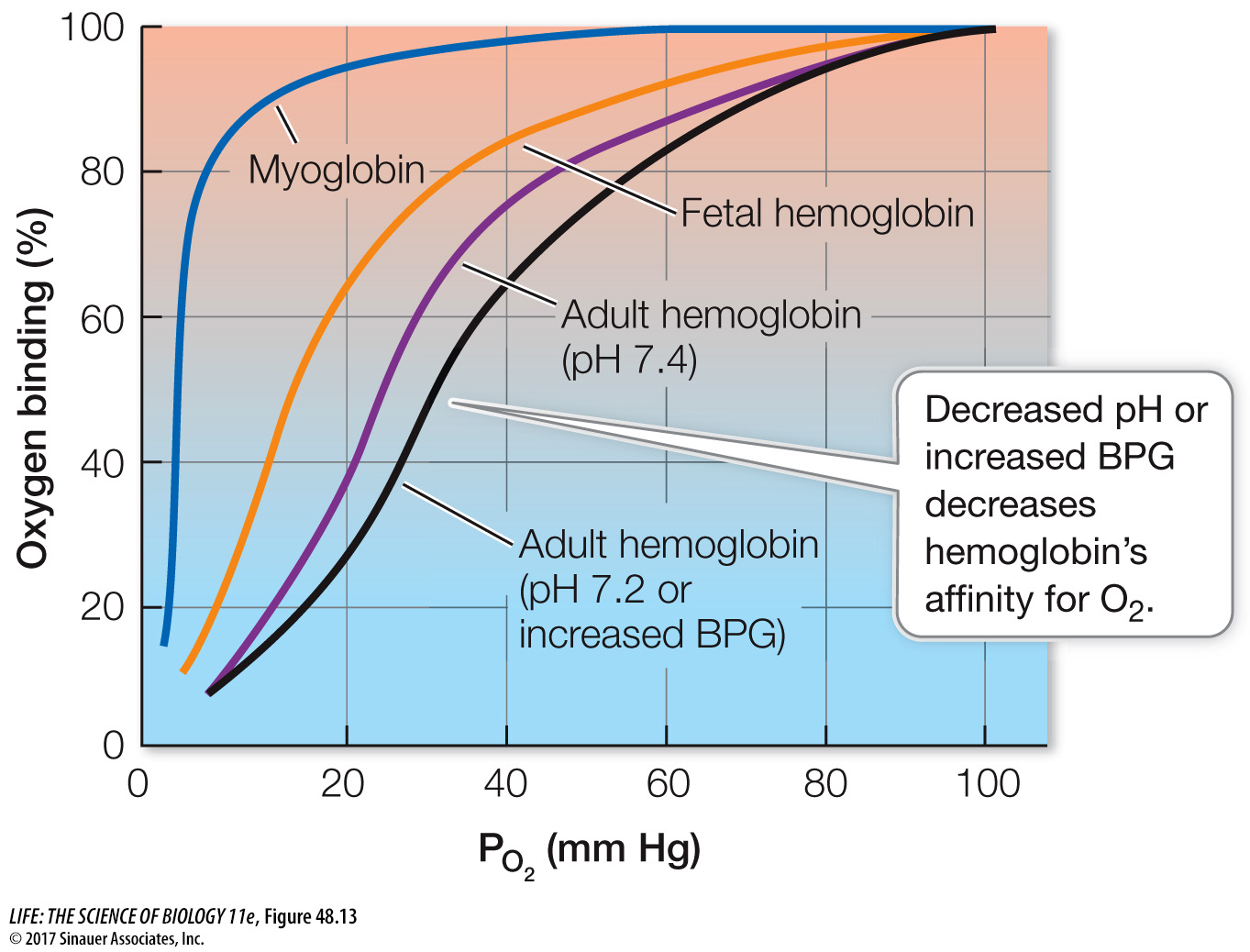
Myoglobin holds an O2 reserve
Muscle cells have their own O2-binding molecule, myoglobin. Myoglobin consists of just one polypeptide chain associated with an iron-
Q: How would the breathing of some carbon monoxide affect the hemoglobin–
The hemoglobin would not fully saturate with O2 at 100 mm Hg PO2.
Activity 48.3 Oxygen-
*connect the concepts The evolutionary relationships between myoglobin and the various hemoglobin subunits is shown in Figure 23.10.
Myoglobin facilitates the diffusion of O2 in muscle cells and provides an O2 reserve for times when metabolic demands are high and blood flow is interrupted. Interruption of blood flow in muscles is common because contracting muscles squeeze blood vessels. When tissue PO2 values are low and hemoglobin can no longer supply more O2, myoglobin releases its bound O2. Diving mammals such as seals have high concentrations of myoglobin in their muscles, which is one reason they can stay under water for so long (Investigating Life: Seals Are Champion Breath-Hold Divers). Even in non-