Most CO2 is transported as bicarbonate ions in the blood
Delivering O2 to tissues is only half the respiratory function of blood. Blood also carries CO2, a metabolic waste product, away from tissues (Figure 48.14). CO2 is highly soluble and readily diffuses through cell membranes, moving from its site of production in the tissues into the blood, where PCO2 is lower. However, only about 5 percent of the CO2 carried by the blood is dissolved CO2. About 20 percent of the CO2 carried to the lungs in the blood is in chemical combination with hemoglobin. And most CO2 produced by the tissues is transported to the lungs in the form of bicarbonate ions (HCO3–). CO2 diffusing from cells is converted to HCO3–, transported to the lungs, and then converted back to CO2 to be exhaled.
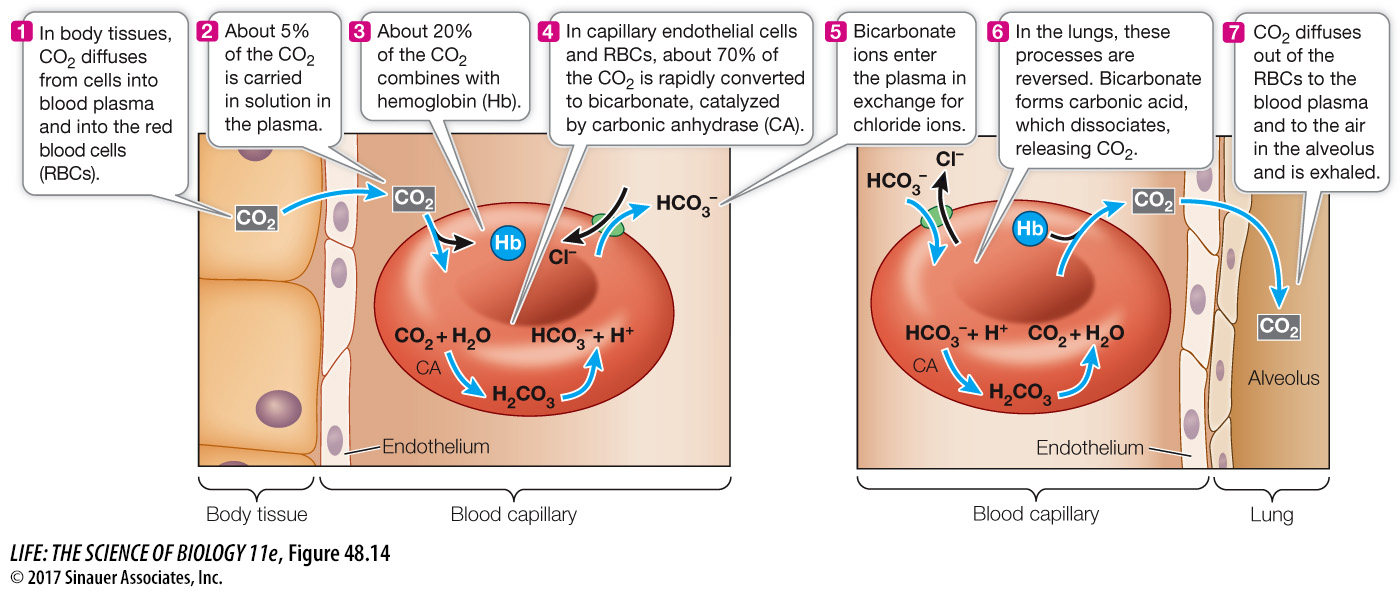
When CO2 dissolves in water, some of it slowly reacts with the water molecules to form carbonic acid (H2CO3), some of which then dissociates into a proton (H+) and a bicarbonate ion (HCO3–). This reversible reaction is expressed as follows:
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3–
In the extracellular fluid, the reaction between CO2 and H2O proceeds slowly. But it is a different story in the endothelial cells of the capillaries and in the RBCs, where the enzyme carbonic anhydrase speeds up the conversion of CO2 to H2CO3. The newly formed H2CO3 dissociates, and the resulting bicarbonate ions enter the plasma in exchange for Cl– (see Figure 48.14). By converting CO2 to H2CO3, carbonic anhydrase reduces the PCO2 in these cells and in the plasma, facilitating the diffusion of CO2 from tissue cells to endothelial cells, plasma, and RBCs.
In the lungs, the reactions involving CO2 and bicarbonate ions are reversed. Remember that an enzyme such as carbonic anhydrase only speeds up a reversible reaction; it does not determine its direction. The direction is determined by concentrations of reactants and products. Ventilation keeps the PCO2 in the alveoli low, so CO2 diffuses from the blood plasma into the alveoli, lowering the PCO2 in the blood, which favors the conversion of HCO3– into CO2. Also, the oxygenation of hemoglobin in the alveoli decreases its affinity for CO2, a phenomenon known as the Haldane effect.