Air is a better respiratory medium than water
The slow diffusion of O2 molecules in water affects both air-
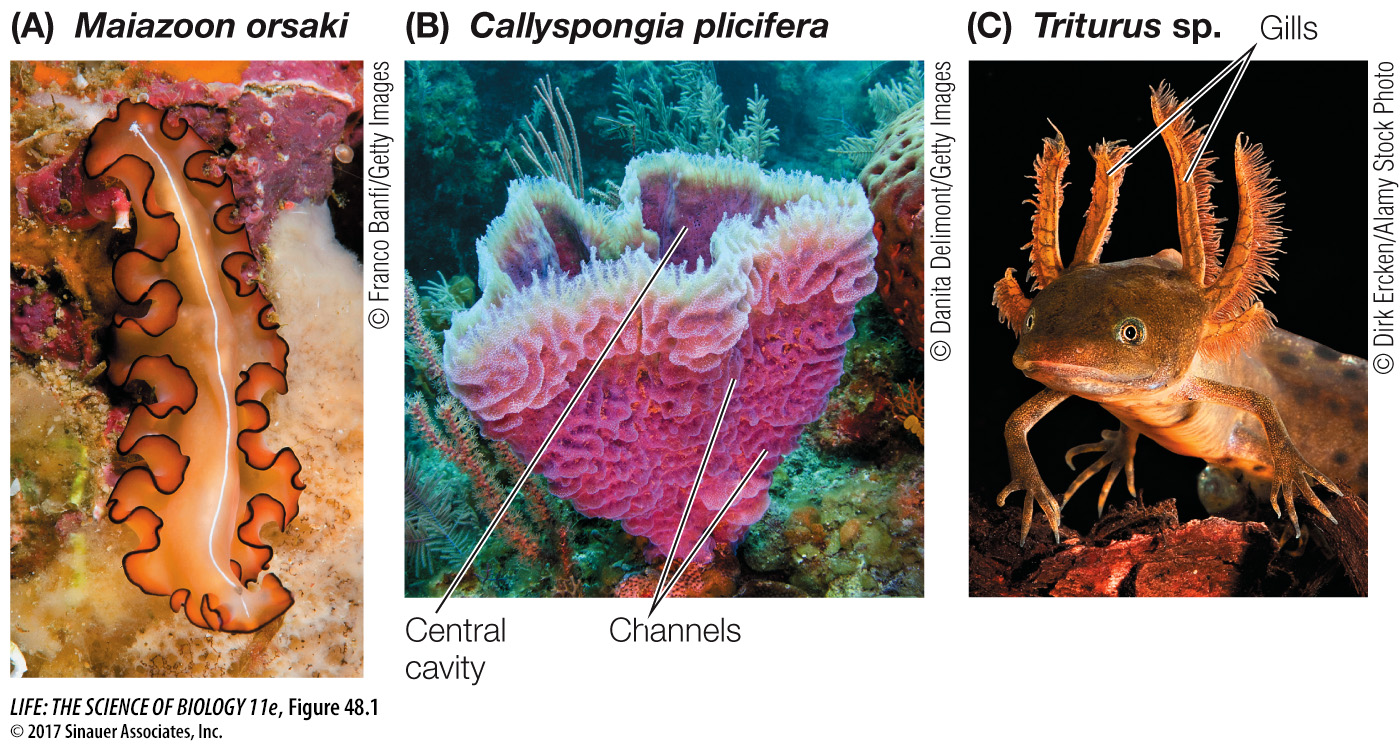
Diffusion of O2 in water is so slow that even animal cells with low rates of metabolism cannot function more than a few millimeters away from a good source of environmental O2. Therefore there are severe size and shape limits on the many species of invertebrates that lack internal systems for distributing O2. Most of these species are very small, but some, such as marine flatworms, have grown larger by evolving a flat, thin body with a large external surface area (Figure 48.1A). Another strategy is seen in sponges, which have bodies made of cells that surround water channels through which the external medium flows (Figure 48.1B) (see also Figure 30.2B). A critical factor enabling larger, more complex animal bodies has been the evolution of specialized respiratory systems with large surface areas such as gills that are highly permeable to respiratory gases (Figure 48.1C).
O2 can be obtained more easily from air than from water for several reasons:
The O2 content of air is much higher than the O2 content of an equal volume of water. The maximum O2 content of a bubbling stream in equilibrium with air is less than 10 milliliters (mL) of O2 per liter of water. The O2 content of the air over the stream is about 200 mL of O2 per liter of air.
O2 diffuses about 8,000 times more rapidly in air than in water. That is why the O2 content of a stagnant pond can be zero only a few millimeters below the surface.
An animal has to work (expend energy) to ventilate its gas exchange surfaces with water or air. More energy is required to move water than air because water is 800 times denser than air and about 50 times more viscous.
You can appreciate how important these facts were for the evolutionary transition of life to the terrestrial environment, because they meant that there were fewer constraints on the evolution of higher metabolic rates.