Chapter 6
RECAP 6.1
The hydrophilic “heads” of fatty acids are the polar ends, and the hydrophobic “tails” are the nonpolar ends. So the heads tend to associate with water molecules, and the tails away from water molecules. Placed in an aqueous environment, the fatty acids will arrange themselves so that their tails interact with one another while their heads face the water of the environment and cytoplasm, forming a bilayer.
An integral membrane protein is embedded in the phospholipid bilayer by hydrophobic interactions with the lipid interior. It must have amino acids with hydrophobic R groups to insert into the nonpolar fatty acid tail region of the membrane bilayer. A peripheral membrane protein lacks hydrophobic regions and does not interact with the hydrophobic core of the phospholipid bilayer. Instead it is usually bound to the membrane indirectly by interactions with integral membrane proteins or directly by interactions with lipid polar head groups.
Both freeze-
fracturing and cell fusion experiments indicate that nonpolar membrane proteins are inserted into the hydrophobic interior of the lipid bilayer. Cell fusion experiments also show that the proteins can move in the plane of the membrane. To measure membrane fluidity, label a small amount of a lipid or protein with a dye and allow it to incorporate into the membrane of a cancer cell and a noncancer cell. This may make a localized labeled spot on the cells. The localized region will be seen to diffuse over the cells over time. In the cancer cell, this rate of diffusion may be faster.
RECAP 6.2
Enzymes called glycosidases cut the bonds between carbohydrates and other molecules. Obtain cells that are bound together (e.g., sponges) and then separate them. Then treat the cells with glycosidases to remove carbohydrates. This should block cell recognition and adhesion.
Plasmodesmata are most similar to gap junctions, because both have a membrane-
lined channel.
RECAP 6.3
The properties that affect diffusion across a membrane are size and mass (smaller is faster), electric charge (less polar is faster), and concentration gradient (the higher the gradient, the faster the rate of diffusion).
If blood is hypotonic, water will enter red blood cells, causing them to swell and perhaps burst. (This is called hemolytic anemia.)
The hydrophobic cell membrane lipids are relatively impermeable to charged ions such as K+. A channel will allow diffusion of K+ out of the cell until equilibrium is reached, with equal concentrations inside and outside the cell.
RECAP 6.4
Substances tend to diffuse and reach equilibrium, where they are evenly distributed in the environment. Energy is needed to overcome this natural tendency.
An antiporter transports two substances in opposite directions. In the case of the Na+–K+ pump, Na+ is transported across the cell membrane outward, and K+ is transported across the cell membrane inward.
In primary active transport, ATP hydrolysis supplies the energy needed for transport against a concentration gradient. In secondary active transport, the energy comes from a gradient set up by a different (secondary) active transport.
Both active transport and facilitated diffusion speed up transport across a membrane by using a protein that binds to the substance transported. However, active transport is an energy-
requiring process that transports a substance against its concentration gradient and diffusion tendency. By contrast, facilitated diffusion does not require energy and transports a substance along its concentration gradient and in conjunction with its tendency to diffuse.
RECAP 6.5
Phagocytosis involves a large cell membrane–
derived vesicle forming around macromolecules. Pinocytosis involves smaller vesicles forming around water and its dissolved solutes. In receptor-
mediated endocytosis, a molecule binds to a cell membrane protein receptor and causes the membrane to form a vesicle around the molecule and receptor. The vesicle with its contents enters the cell and usually fuses with a lysosome. An example is the endocytosis of lipoprotein particles from blood into liver cells. Diatom wall components move from the Golgi apparatus to the cell wall by exocytosis.
WORK WITH THE DATA, P. 122
The mRNA-
injected oocytes swelled because of osmotic uptake of water. At 4 minutes, the mRNA- injected cells had taken up so much water that they burst. The control cells did not take up excess water and therefore stayed intact. 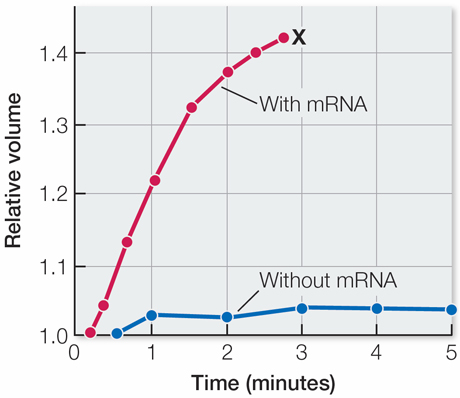
Water permeability increased with more mRNA injected, presumably because there was more aquaporin in the membranes that had more mRNA. The relationship could be evaluated statistically by linear regression.
The data on the mRNA-
injected oocytes for mercuric chloride alone showed reduced water permeability, indicating that a protein was involved. Adding mercaptoethanol restored water permeability. There was not much water permeability in the controls without added mRNA, and mercuric chloride and mercaptoethanol had no effect on this.
FIGURE QUESTIONS
Figure 6.1 Hydrophobic interactions keep some proteins embedded whereas ionic attractions keep others on the surface.
Figure 6.10 Overfertilizing makes the soil water hypertonic to the interior of the plant root cells. Water leaves the plant roots by osmosis, moving toward the hypertonic medium. Water in the plant organs also travels to the roots, where it leaves by osmosis. Because cellular water is important in maintaining turgor pressure, the loss of water resulting from overfertilization causes the plant to wilt.
Figure 6.15 Blocking the Na+–K+ pump will result in equal concentrations of Na+ inside and outside the cell. Generally, this will increase the cellular Na+. The absence of a Na+ gradient will mean there is no potential energy to drive glucose uptake into the cell, so the glucose concentration will be reduced.
APPLY WHAT YOU’VE LEARNED
When measured at a constant temperature, the cell membranes show a trend toward greater fluidity with decreasing temperature of a species’ native environment. The least fluid membranes are found in animals that experience the highest temperatures (rats with body temperatures of 37°C). Fluidity is slightly greater in desert pupfish at 34°C and increases even more in goldfish raised at 25°C. This trend continues with increasing fluidity in goldfish raised at 5°C. The greatest fluidity is observed in arctic sculpin raised in extremely cold temperatures (0°C).
The data suggest that the ratio of saturated to unsaturated fatty acids in phospholipids making up the membrane also influences its fluidity.
Saturated fatty acids can pack together more tightly than unsaturated fatty acids, reducing membrane fluidity. Shifting to a greater proportion of unsaturated fatty acids would be appropriate as temperatures decrease, because molecular motion decreases as temperature decreases. At very low temperatures, the membrane needs more unsaturated fatty acids to reduce tight packing and maintain fluidity. At very high temperatures, the membrane can accommodate tight-
packing saturated fatty acids because molecular motion is greater. From the fluorescence value, the graph indicates that the cell membranes of the animal must have been raised at a temperature of about 15°C. This temperature falls between data collected for goldfish at 5°C and 25°C. This means that the ratio of saturated to unsaturated fatty acids in this animal might be expected to fall somewhere between 0.66 and 0.82 based on the data provided in the table, and probably close to 0.70.